The meso-connectomes of mouse, marmoset, and macaque: network organization and the emergence of higher cognition
- PMID: 38771244
- PMCID: PMC11107384
- DOI: 10.1093/cercor/bhae174
The meso-connectomes of mouse, marmoset, and macaque: network organization and the emergence of higher cognition
Abstract
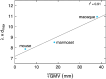
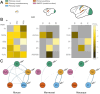
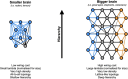
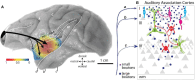
The recent publications of the inter-areal connectomes for mouse, marmoset, and macaque cortex have allowed deeper comparisons across rodent vs. primate cortical organization. In general, these show that the mouse has very widespread, "all-to-all" inter-areal connectivity (i.e. a "highly dense" connectome in a graph theoretical framework), while primates have a more modular organization. In this review, we highlight the relevance of these differences to function, including the example of primary visual cortex (V1) which, in the mouse, is interconnected with all other areas, therefore including other primary sensory and frontal areas. We argue that this dense inter-areal connectivity benefits multimodal associations, at the cost of reduced functional segregation. Conversely, primates have expanded cortices with a modular connectivity structure, where V1 is almost exclusively interconnected with other visual cortices, themselves organized in relatively segregated streams, and hierarchically higher cortical areas such as prefrontal cortex provide top-down regulation for specifying precise information for working memory storage and manipulation. Increased complexity in cytoarchitecture, connectivity, dendritic spine density, and receptor expression additionally reveal a sharper hierarchical organization in primate cortex. Together, we argue that these primate specializations permit separable deconstruction and selective reconstruction of representations, which is essential to higher cognition.
Keywords: cortex; hippocampus; primate; rodent; working memory.
© The Author(s) 2024. Published by Oxford University Press.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

