Hydrophobic residues in S1 modulate enzymatic function and voltage sensing in voltage-sensing phosphatase
- PMID: 38771271
- PMCID: PMC11109755
- DOI: 10.1085/jgp.202313467
Hydrophobic residues in S1 modulate enzymatic function and voltage sensing in voltage-sensing phosphatase
Abstract
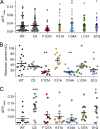
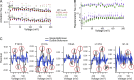
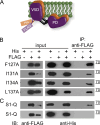
The voltage-sensing domain (VSD) is a four-helix modular protein domain that converts electrical signals into conformational changes, leading to open pores and active enzymes. In most voltage-sensing proteins, the VSDs do not interact with one another, and the S1-S3 helices are considered mainly scaffolding, except in the voltage-sensing phosphatase (VSP) and the proton channel (Hv). To investigate its contribution to VSP function, we mutated four hydrophobic amino acids in S1 to alanine (F127, I131, I134, and L137), individually or in combination. Most of these mutations shifted the voltage dependence of activity to higher voltages; however, not all substrate reactions were the same. The kinetics of enzymatic activity were also altered, with some mutations significantly slowing down dephosphorylation. The voltage dependence of VSD motions was consistently shifted to lower voltages and indicated a second voltage-dependent motion. Additionally, none of the mutations broke the VSP dimer, indicating that the S1 impact could stem from intra- and/or intersubunit interactions. Lastly, when the same mutations were introduced into a genetically encoded voltage indicator, they dramatically altered the optical readings, making some of the kinetics faster and shifting the voltage dependence. These results indicate that the S1 helix in VSP plays a critical role in tuning the enzyme's conformational response to membrane potential transients and influencing the function of the VSD.
© 2024 Rayaprolu et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







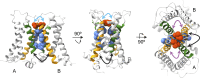
Update of
-
S1 hydrophobic residues modulate voltage sensing phosphatase enzymatic function and voltage sensing.bioRxiv [Preprint]. 2024 Apr 9:2023.12.27.573443. doi: 10.1101/2023.12.27.573443. bioRxiv. 2024. Update in: J Gen Physiol. 2024 Jul 1;156(7):e202313467. doi: 10.1085/jgp.202313467. PMID: 38234747 Free PMC article. Updated. Preprint.
Similar articles
-
S1 hydrophobic residues modulate voltage sensing phosphatase enzymatic function and voltage sensing.bioRxiv [Preprint]. 2024 Apr 9:2023.12.27.573443. doi: 10.1101/2023.12.27.573443. bioRxiv. 2024. Update in: J Gen Physiol. 2024 Jul 1;156(7):e202313467. doi: 10.1085/jgp.202313467. PMID: 38234747 Free PMC article. Updated. Preprint.
-
Tuning the voltage-sensor motion with a single residue.Biophys J. 2012 Aug 8;103(3):L23-L25. doi: 10.1016/j.bpj.2012.06.030. Biophys J. 2012. PMID: 22947880 Free PMC article.
-
Interaction between S4 and the phosphatase domain mediates electrochemical coupling in voltage-sensing phosphatase (VSP).Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2200364119. doi: 10.1073/pnas.2200364119. Epub 2022 Jun 21. Proc Natl Acad Sci U S A. 2022. PMID: 35733115 Free PMC article.
-
Dynamic structural rearrangements and functional regulation of voltage-sensing phosphatase.J Physiol. 2019 Jan;597(1):29-40. doi: 10.1113/JP274113. Epub 2018 Nov 22. J Physiol. 2019. PMID: 30311949 Free PMC article. Review.
-
Voltage-Sensing Phosphatases: Biophysics, Physiology, and Molecular Engineering.Physiol Rev. 2018 Oct 1;98(4):2097-2131. doi: 10.1152/physrev.00056.2017. Physiol Rev. 2018. PMID: 30067160 Review.
Cited by
-
Voltage clamp fluorometry in Xenopus laevis oocytes to study the voltage-sensing phosphatase.Bio Protoc. 2025 Feb 20;15(4):e5212. doi: 10.21769/BioProtoc.5212. eCollection 2025 Feb 20. Bio Protoc. 2025. PMID: 40028022 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

