Burden of Disease Study of Patients with Diabetic Macular Oedema in Spain
- PMID: 38771462
- PMCID: PMC11178718
- DOI: 10.1007/s40123-024-00959-2
Burden of Disease Study of Patients with Diabetic Macular Oedema in Spain
Abstract
Introduction: Diabetic macular oedema (DMO) is a complication of diabetic retinopathy that can result in vision loss. The disease can impact different spheres of a patient's life, including physical and psychological health, work, and activities of daily living, entailing an important use of healthcare and non-healthcare resources. This study aimed to estimate the socio-economic burden of DMO in Spain.
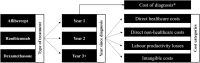
Methods: The burden of DMO was estimated from a societal perspective, per patient, year of treatment since diagnosis, and type of treatment. Four categories were considered: direct healthcare costs (DHC), direct non-healthcare costs (DNHC), labour productivity losses (LPL), and intangible costs (IC) associated with loss of quality of life. Average annual costs were calculated by multiplying the resources used per patient by their corresponding unit price (or financial proxy). For a more accurate estimation, differences in resource use between treatments (intravitreal anti-vascular endothelial growth factor injections of ranibizumab or aflibercept, and intravitreal dexamethasone implants) and year since diagnosis (first, second, and third year or beyond) were considered and presented separately. The reference year for costs was 2021.
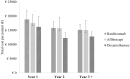
Results: The average annual costs of DMO in the first year of treatment after diagnosis was estimated at €18,774, €17,512, and €16,188 per patient treated with ranibizumab, aflibercept, and dexamethasone, respectively. This burden would be reduced to €15,783, €15,701, and €12,233 in the second year, and to €15,119, €15,043, and €12,790 in the third year, respectively. Diagnosis of DMO entails an additional one-off cost of €485. DHC accounted for the greatest proportion of total annual costs per patient, independent of the year, with LPL also making an important contribution to total costs.
Conclusions: The socio-economic impact of DMO on patients, the healthcare system, and society at large is substantial. The constant increase in its prevalence accentuates the need for planning and implementation of healthcare strategies to prevent vision loss and reduce the socio-economic burden of the disease.
Keywords: Burden of disease; DMO; Healthcare costs; Non-healthcare costs; Productivity losses; Quality of life; Resource consumption.
© 2024. The Author(s).
Conflict of interest statement
José M. Ruiz-Moreno, María Gámez Lechuga, Pilar Calvo, and Maximino J. Abraldes declare having received payments from Roche for participating as members of the advisory committee for this study. María Merino, Teresa Martín Lorenzo, and Paulina Maravilla-Herrera declare that they are employees of Weber, a company that has received payments from Roche to conduct this study. Beatriz Gil Jiménez declares to be an employee of Roche Farma, a company that has funded this study.
Figures

Similar articles
-
Burden of Disease Study of Patients with Neovascular Age-Related Macular Degeneration in Spain.Ophthalmol Ther. 2024 Jul;13(7):1925-1935. doi: 10.1007/s40123-024-00960-9. Epub 2024 May 21. Ophthalmol Ther. 2024. PMID: 38771461 Free PMC article.
-
Analysis of costs associated with the management and morbidity of diabetic macular oedema and macular oedema secondary to retinal vein occlusion.Arch Soc Esp Oftalmol. 2016 Jun;91(6):273-80. doi: 10.1016/j.oftal.2015.11.019. Epub 2016 Jan 19. Arch Soc Esp Oftalmol. 2016. PMID: 26810908 English, Spanish.
-
Healthcare expenditure of intravitreal anti-vascular endothelial growth factor inhibitors compared with dexamethasone implant for diabetic macular oedema.Acta Ophthalmol. 2022 Dec;100(8):e1630-e1640. doi: 10.1111/aos.15151. Epub 2022 Apr 25. Acta Ophthalmol. 2022. PMID: 35467793 Free PMC article.
-
Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis.BMJ Open. 2019 May 28;9(5):e022031. doi: 10.1136/bmjopen-2018-022031. BMJ Open. 2019. PMID: 31142516 Free PMC article.
-
A review of therapies for diabetic macular oedema and rationale for combination therapy.Eye (Lond). 2015 Sep;29(9):1115-30. doi: 10.1038/eye.2015.110. Epub 2015 Jun 26. Eye (Lond). 2015. PMID: 26113500 Free PMC article. Review.
Cited by
-
Twelve-Month Outcomes of Faricimab for Patients with Sub-Optimally Responsive Diabetic Macular Oedema: A Retrospective Single-Centre Study.Clin Ophthalmol. 2025 May 16;19:1583-1591. doi: 10.2147/OPTH.S513009. eCollection 2025. Clin Ophthalmol. 2025. PMID: 40396158 Free PMC article.
References
-
- GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–e160. doi: 10.1016/S2214-109X(20)30489-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

