Cost Effectiveness of Fremanezumab in Episodic and Chronic Migraine Patients from a Japanese Healthcare Perspective
- PMID: 38771521
- PMCID: PMC11180151
- DOI: 10.1007/s40273-024-01380-0
Cost Effectiveness of Fremanezumab in Episodic and Chronic Migraine Patients from a Japanese Healthcare Perspective
Abstract
Background and objectives: Fremanezumab is an effective treatment for episodic (EM) and chronic migraine (CM) patients in Japan, but its cost effectiveness remains unknown. The objective of this study was to determine the cost effectiveness of fremanezumab compared with standard of care (SOC) in previously treated EM and CM patients from a Japanese healthcare perspective.
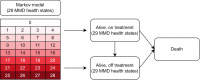
Methods: Estimated regression models were implemented in a probabilistic Markov model to inform effectiveness and health-related quality-of-life data for fremanezumab and SOC. The model was further populated with data from the literature. The adjusted Japanese healthcare perspective included productivity losses. The main model outcomes were quality-adjusted life-years (QALYs), costs (2022 Japanese Yen [¥]), and incremental outcomes including the incremental cost-effectiveness ratio (ICER). Analyses were performed separately for the EM and CM patients and combined. Costs and effects were discounted at an annual rate of 2.0%.
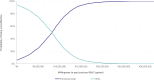
Results: The mean QALYs over a 25-year time horizon for the EM and CM populations combined were 13.03 for SOC and 13.15 for fremanezumab. The associated costs were ¥27,550,292 for SOC and ¥28,371,048 for fremanezumab. QALYs were higher and costs lower for EM patients compared with CM patients for both fremanezumab and SOC. The deterministic ICERs of fremanezumab versus SOC were ¥6,334,861 for EM, ¥7,393,824 for CM, and ¥6,530,398 for EM and CM combined. Indirect costs and choice of mean migraine days model distribution had a substantial impact on the ICER.
Conclusion: Using fremanezumab in a heterogeneous mixture of Japanese EM and CM patients resulted in a reduction of monthly migraine days and thus more QALYs compared with SOC. The cost effectiveness of fremanezumab versus SOC in EM and CM patients resulted in an ICER of ¥6,530,398, from an adjusted Japanese public healthcare perspective.
Plain language summary
Fremanezumab is an effective treatment for episodic and chronic migraine patients in Japan, but it is unknown how the costs relate to the health benefits. The current research determined the relation between costs and effects of fremanezumab compared with the current standard of care in Japanese clinical practice, to see if the costs are justified by the health benefits. A model was used to inform the treatment effect of fremanezumab and standard of care. Data on costs, the frequency in which health care was used, and impairment of work due to migraine were also included in the model and obtained from the literature. The main outcomes were the number of years that patients were alive while taking their quality of life into account, costs, and the difference in these outcomes between patients who were treated with fremanezumab and those receiving standard of care. Subsequently, it was estimated how costs and effects related to one another and whether the costs were justified by the health benefits. The outcomes showed that patients treated with fremanezumab had a better quality of life compared with those receiving standard of care, while the costs associated with fremanezumab were higher. Compared with standard of care, the health benefits of treating patients with fremanezumab were justified by the costs within an acceptable range. Taking the absence from work due to illness into account had a substantial impact on the model outcomes.
© 2024. The Author(s).
Conflict of interest statement
Takao Takeshima reports consulting fees or honorarium by Otsuka, Amgen, Eli-Lilly, Daiichi Sankyo, Shionogi, Bioheaven, and Lundbeck and being an advisor of Hedgehog MedTech and Sawai. Fumihiko Sakai reports consulting fees or honorarium by Otsuka, Amgen, Eli-Lilly, and Daiichi Sankyo. Xinyu Wang was employed by Otsuka Pharmaceutical Co., Ltd. during the conduct of the study and reports no conflicts of interest. Kentaro Yamato, Yoshitsugu Kojima, and Yilong Zhang are employed by Otsuka Pharmaceutical Co., Ltd. and report no conflicts of interest. Otsuka Pharmaceutical Co., Ltd. launched fremanezumab in Japan. Craig Bennison and Martijn Simons are employed by OPEN Health and report consulting fees from Otsuka. OPEN Health is a consultancy firm that was contracted by Otsuka to conduct the study and write the manuscript.
Figures

References
-
- Japanese Society of Neurology and the Japanese Headache Society. Clinical practice guideline for chronic headache. https://www.neurology-jp.org/guidelinem/ch/index.html. Accessed 22 Apr 22 2020. 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

