β-Blocker Use and Clinical Outcomes in Patients With COPD Following Acute Myocardial Infarction
- PMID: 38771577
- PMCID: PMC11109775
- DOI: 10.1001/jamanetworkopen.2024.7535
β-Blocker Use and Clinical Outcomes in Patients With COPD Following Acute Myocardial Infarction
Abstract
Importance: While β-blockers are associated with decreased mortality in cardiovascular disease (CVD), exacerbation-prone patients with chronic obstructive pulmonary disease (COPD) who received metoprolol in the Beta-Blockers for the Prevention of Acute Exacerbations of Chronic Obstructive Pulmonary Disease (BLOCK-COPD) trial experienced increased risk of exacerbations requiring hospitalization. However, the study excluded individuals with established indications for the drug, raising questions about the overall risk and benefit in patients with COPD following acute myocardial infarction (AMI).
Objective: To investigate whether β-blocker prescription at hospital discharge is associated with increased risk of mortality or adverse cardiopulmonary outcomes in patients with COPD and AMI.
Design, setting, and participants: This prospective, longitudinal cohort study with 6 months of follow-up enrolled patients aged 35 years or older with COPD who underwent cardiac catheterization for AMI at 18 BLOCK-COPD network hospitals in the US from June 2020 through May 2022.
Exposure: Prescription for any β-blocker at hospital discharge.
Main outcomes and measures: The primary outcome was time to the composite outcome of death or all-cause hospitalization or revascularization. Secondary outcomes included death, hospitalization, or revascularization for CVD events, death or hospitalization for COPD or respiratory events, and treatment for COPD exacerbations.
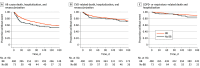
Results: Among 3531 patients who underwent cardiac catheterization for AMI, prevalence of COPD was 17.1% (95% CI, 15.8%-18.4%). Of 579 total patients with COPD and AMI, 502 (86.7%) were prescribed a β-blocker at discharge. Among the 562 patients with COPD included in the final analysis, median age was 70.0 years (range, 38.0-94.0 years) and 329 (58.5%) were male; 553 of the 579 patients (95.5%) had follow-up information. Among those discharged with β-blockers, there was no increased risk of the primary end point of all-cause mortality, revascularization, or hospitalization (hazard ratio [HR], 1.01; 95% CI, 0.66-1.54; P = .96) or of cardiovascular events (HR, 1.11; 95% CI, 0.65-1.92; P = .69), COPD-related or respiratory events (HR, 0.75; 95% CI, 0.34-1.66; P = .48), or treatment for COPD exacerbations (rate ratio, 1.01; 95% CI, 0.53-1.91; P = .98).
Conclusions and relevance: In this cohort study, β-blocker prescription at hospital discharge was not associated with increased risk of adverse outcomes in patients with COPD and AMI. These findings support use of β-blockers in patients with COPD and recent AMI.
Conflict of interest statement
Figures

References
-
- GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736-1788. doi: 10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

