Protein N -Glycans in Healthy and Sclerotic Glomeruli in Diabetic Kidney Disease
- PMID: 38771634
- PMCID: PMC11387035
- DOI: 10.1681/ASN.0000000000000393
Protein N -Glycans in Healthy and Sclerotic Glomeruli in Diabetic Kidney Disease
Abstract
Key Points:
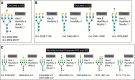
Multiomics performed on diabetic kidney disease biopsies revealed five N-glycan signatures of sclerotic glomeruli that significantly differed compared with healthy glomeruli.
Integrative spatial glycomics, proteomics, and transcriptomics revealed protein N-glycosylation characteristic of sclerotic glomeruli in diabetic kidney disease.
Background: Diabetes is expected to directly affect renal glycosylation; yet to date, there has not been a comprehensive evaluation of alterations in N-glycan composition in the glomeruli of patients with diabetic kidney disease (DKD).
Methods: We used untargeted mass spectrometry imaging to identify N-glycan structures in healthy and sclerotic glomeruli in formalin-fixed paraffin-embedded sections from needle biopsies of five patients with DKD and three healthy kidney samples. Regional proteomics was performed on glomeruli from additional biopsies from the same patients to compare the abundances of enzymes involved in glycosylation. Secondary analysis of single-nucleus RNA sequencing (snRNAseq) data were used to inform on transcript levels of glycosylation machinery in different cell types and states.
Results: We detected 120 N-glycans, and among them, we identified 12 of these protein post-translated modifications that were significantly increased in glomeruli. All glomeruli-specific N-glycans contained an N-acetyllactosamine epitope. Five N-glycan structures were highly discriminant between sclerotic and healthy glomeruli. Sclerotic glomeruli had an additional set of glycans lacking fucose linked to their core, and they did not show tetra-antennary structures that were common in healthy glomeruli. Orthogonal omics analyses revealed lower protein abundance and lower gene expression involved in synthesizing fucosylated and branched N-glycans in sclerotic podocytes. In snRNAseq and regional proteomics analyses, we observed that genes and/or proteins involved in sialylation and N-acetyllactosamine synthesis were also downregulated in DKD glomeruli, but this alteration remained undetectable by our spatial N-glycomics assay.
Conclusions: Integrative spatial glycomics, proteomics, and transcriptomics revealed protein N-glycosylation characteristic of sclerotic glomeruli in DKD.
Keywords: diabetic glomerulosclerosis; diabetic kidney disease.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures




References
Grants and funding
- UH3 DK114907/DK/NIDDK NIH HHS/United States
- UH3 DK114923/DK/NIDDK NIH HHS/United States
- U2C DK114886/DK/NIDDK NIH HHS/United States
- UH3 DK114920/DK/NIDDK NIH HHS/United States
- U01 DK114908/DK/NIDDK NIH HHS/United States
- UH3 DK114937/DK/NIDDK NIH HHS/United States
- U01 DK114920/DK/NIDDK NIH HHS/United States
- UH3 DK114933/DK/NIDDK NIH HHS/United States
- UH3 DK114923/DK/NIDDK NIH HHS/United States
- UH3 DK114920/DK/NIDDK NIH HHS/United States
- UH3 DK114933/DK/NIDDK NIH HHS/United States
- UH3 DK114937/DK/NIDDK NIH HHS/United States
- UH3 DK114907/DK/NIDDK NIH HHS/United States
- U2C DK114886/DK/NIDDK NIH HHS/United States
- UH3 DK114923/DK/NIDDK NIH HHS/United States
- UH3 DK114920/DK/NIDDK NIH HHS/United States
- UH3 DK114933/DK/NIDDK NIH HHS/United States
- UH3 DK114937/DK/NIDDK NIH HHS/United States
- UH3 DK114907/DK/NIDDK NIH HHS/United States
- U2C DK114886/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

