Safety and efficacy of continuous terlipressin infusion in HRS-AKI in a transplant population
- PMID: 38771635
- PMCID: PMC11398294
- DOI: 10.1097/LVT.0000000000000399
Safety and efficacy of continuous terlipressin infusion in HRS-AKI in a transplant population
Abstract
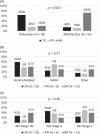
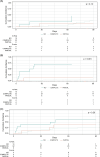
Hepatorenal syndrome-acute kidney injury (HRS-AKI) is associated with significant morbidity and mortality. While liver transplantation is the definitive treatment, continuous terlipressin infusion for HRS-AKI may provide benefit and, as such, was assessed in a population composed of candidates for liver transplant (LT). Fifty hospitalized LT-eligible patients with HRS-AKI received a single bolus followed by continuous terlipressin infusion. Acute-on-chronic liver failure grade 3, serum creatinine (SCr)>5.0 mg/dL, or Model for End-Stage Liver Disease (MELD) ≥35 were exclusions. Fifty hospitalized patients who received midodrine and octreotide or norepinephrine for HRS-AKI served as a historical comparator cohort. Complete response (CR) was defined as a ≥30% decrease in SCr with end-of-treatment (EOT) SCr≤1.5, partial response as a ≥30% decrease in SCr with EOT SCr>1.5, and nonresponse as a <30% decrease in SCr. CR rate was significantly higher in the terlipressin cohort compared to the historical cohort (64% vs. 16%, p <0.001). Survival, while numerically higher in those who received terlipressin, was statistically similar (D30: 94% vs. 82%, p =0.12; D90: 78% vs. 68%, p =0.37). Renal replacement therapy (RRT) was more common among terlipressin NR than CR and PR (70% vs. 3% vs. 13%, p < 0.001). EOT MELD and SCr were significantly lower within terlipressin cohort (MELD: 19 vs. 25, SCr: 1.4 vs. 2.1 mg/dL, p <0.001). Sixteen of 40 terlipressin-treated patients received LT-alone (terlipressin CR in 10/16). One patient on terlipressin had a hypoxic respiratory failure that responded to diuretics; one possibly had drug-related rash. With continuous terlipressin infusion, a CR rate of 64% was observed with a favorable safety profile. Terlipressin use was associated with lower EOT MELD and SCr than the historical midodrine and octreotide/norepinephrine cohort; LT-alone was accomplished in a high proportion of complete terlipressin responders.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
K. Rajender Reddy consults for, advises, and received grants from Mallinckrodt. He consults for and received grants from Biovie. He consults for Spark, Novo Nordisk, and Genfit. He advises Novartis and Astra Zeneca. He received grants from BMS, Intercept, Sequana, Grifols, Exact Sciences, TARGET, and Merck. He receives royalties from UpToDate and is an associate editor for
Figures

References
-
- Biggins SW, Angeli P, Garcia‐Tsao G, Ginès P, Ling SC, Nadim MK, et al. . Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–48. - PubMed
-
- Singal AK, Kuo YF, Reddy KR, Bataller R, Kwo P. Healthcare burden and outcomes of hepatorenal syndrome among cirrhosis-related hospitalisations in the US. Aliment Pharmacol Ther. 2022;56:1486–96. - PubMed
-
- Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:23. - PubMed
-
- Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

