BET bromodomain inhibition potentiates radiosensitivity in models of H3K27-altered diffuse midline glioma
- PMID: 38771655
- PMCID: PMC11213469
- DOI: 10.1172/JCI174794
BET bromodomain inhibition potentiates radiosensitivity in models of H3K27-altered diffuse midline glioma
Abstract
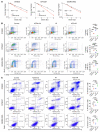
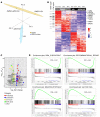
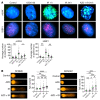
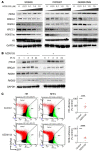
Diffuse midline glioma (DMG) H3K27-altered is one of the most malignant childhood cancers. Radiation therapy remains the only effective treatment yet provides a 5-year survival rate of only 1%. Several clinical trials have attempted to enhance radiation antitumor activity using radiosensitizing agents, although none have been successful. Given this, there is a critical need for identifying effective therapeutics to enhance radiation sensitivity for the treatment of DMG. Using high-throughput radiosensitivity screening, we identified bromo- and extraterminal domain (BET) protein inhibitors as potent radiosensitizers in DMG cells. Genetic and pharmacologic inhibition of BET bromodomain activity reduced DMG cell proliferation and enhanced radiation-induced DNA damage by inhibiting DNA repair pathways. RNA-Seq and the CUT&RUN (cleavage under targets and release using nuclease) analysis showed that BET bromodomain inhibitors regulated the expression of DNA repair genes mediated by H3K27 acetylation at enhancers. BET bromodomain inhibitors enhanced DMG radiation response in patient-derived xenografts as well as genetically engineered mouse models. Together, our results highlight BET bromodomain inhibitors as potential radiosensitizer and provide a rationale for developing combination therapy with radiation for the treatment of DMG.
Keywords: Brain cancer; Epigenetics; Oncology; Therapeutics; Translation.
Conflict of interest statement
Figures