Matriglycan maintains t-tubule structural integrity in cardiac muscle
- PMID: 38771868
- PMCID: PMC11145246
- DOI: 10.1073/pnas.2402890121
Matriglycan maintains t-tubule structural integrity in cardiac muscle
Abstract
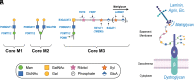
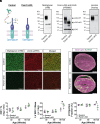
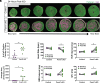
Maintaining the structure of cardiac membranes and membrane organelles is essential for heart function. A critical cardiac membrane organelle is the transverse tubule system (called the t-tubule system) which is an invagination of the surface membrane. A unique structural characteristic of the cardiac muscle t-tubule system is the extension of the extracellular matrix (ECM) from the surface membrane into the t-tubule lumen. However, the importance of the ECM extending into the cardiac t-tubule lumen is not well understood. Dystroglycan (DG) is an ECM receptor in the surface membrane of many cells, and it is also expressed in t-tubules in cardiac muscle. Extensive posttranslational processing and O-glycosylation are required for DG to bind ECM proteins and the binding is mediated by a glycan structure known as matriglycan. Genetic disruption resulting in defective O-glycosylation of DG results in muscular dystrophy with cardiorespiratory pathophysiology. Here, we show that DG is essential for maintaining cardiac t-tubule structural integrity. Mice with defects in O-glycosylation of DG developed normal t-tubules but were susceptible to stress-induced t-tubule loss or severing that contributed to cardiac dysfunction and disease progression. Finally, we observed similar stress-induced cardiac t-tubule disruption in a cohort of mice that solely lacked matriglycan. Collectively, our data indicate that DG in t-tubules anchors the luminal ECM to the t-tubule membrane via the polysaccharide matriglycan, which is critical to transmitting structural strength of the ECM to the t-tubules and provides resistance to mechanical stress, ultimately preventing disruptions in cardiac t-tubule integrity.
Keywords: O-mannosylation; cardiac muscle; dystroglycan; matriglycan; t-tubule.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Klietsch R., Ervasti J. M., Arnold W., Campbell K. P., Jorgensen A. O., Dystrophin-glycoprotein complex and laminin colocalize to the sarcolemma and transverse tubules of cardiac muscle. Circ. Res. 72, 349–360 (1993). - PubMed
-
- Crossman D. J., et al. , Increased collagen within the transverse tubules in human heart failure. Cardiovasc. Res. 113, 879–891 (2017). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

