Final Results of RIGHT Choice: Ribociclib Plus Endocrine Therapy Versus Combination Chemotherapy in Premenopausal Women With Clinically Aggressive Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer
- PMID: 38771995
- PMCID: PMC11315404
- DOI: 10.1200/JCO.24.00144
Final Results of RIGHT Choice: Ribociclib Plus Endocrine Therapy Versus Combination Chemotherapy in Premenopausal Women With Clinically Aggressive Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer
Abstract
Purpose: A head-to-head comparison of efficacy between a cyclin-dependent kinase 4/6 inhibitor plus endocrine therapy (ET) versus combination chemotherapy (CT) has never been reported in patients with clinically aggressive hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer (ABC).
Methods: In this open-label, multicenter, randomized phase II trial, pre/perimenopausal women with clinically aggressive HR+/HER2- ABC were randomly assigned 1:1 to first-line ribociclib (600 mg once daily; 3 weeks on, 1 week off) plus letrozole/anastrozole and goserelin or investigator's choice of combination CT (docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine). The primary end point was progression-free survival (PFS).
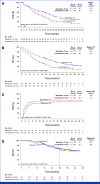
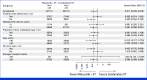
Results: Among 222 patients randomly assigned to ribociclib plus ET (n = 112) or combination CT (n = 110), 150 (67.6%) had symptomatic visceral metastases, 41 (18.5%) had rapid disease progression per investigator's judgment, and 31 (14.0%) had symptomatic nonvisceral disease. Overall, 106 (47.7%) patients had investigator-assessed visceral crisis. The median follow-up time was 37.0 months. At data cutoff, 31.3% (ribociclib arm) and 15.5% (CT arm) of patients had completed study treatment and transitioned to post-trial access. The median PFS was 21.8 months (ribociclib plus ET; [95% CI, 17.4 to 26.7]) and 12.8 months (combination CT; [95% CI, 10.1 to 18.4); hazard ratio, 0.61 [95% CI, 0.43 to 0.87]; P = .003. The overall response rates and the median time to response in the ribociclib versus CT arms, respectively, were 66.1% and 61.8% and 4.9 months and 3.2 months (hazard ratio, 0.76 [95% CI, 0.55 to 1.06]). Lower rates of symptomatic adverse events were observed in the ribociclib versus CT arm.
Conclusion: First-line ribociclib plus ET showed a significant PFS benefit, similar response rates, and better tolerability over combination CT in patients with clinically aggressive HR+/HER2- ABC.
Trial registration: ClinicalTrials.gov NCT03839823.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- El Saghir NS, Khalil MK, Eid T, et al. : Trends in epidemiology and management of breast cancer in developing Arab countries: A literature and registry analysis. Int J Surg 5:225-233, 2007 - PubMed
-
- Bardia A, Hurvitz S: Targeted therapy for premenopausal women with HR+, HER2− advanced breast cancer: Focus on special considerations and latest advances. Clin Cancer Res 24:5206-5218, 2018 - PubMed
-
- Heer E, Harper A, Escandor N, et al. : Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob Health 8:e1027-e1037, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

