Multinuclear non-heme iron dependent oxidative enzymes (MNIOs) involved in unusual peptide modifications
- PMID: 38772214
- PMCID: PMC11806912
- DOI: 10.1016/j.cbpa.2024.102467
Multinuclear non-heme iron dependent oxidative enzymes (MNIOs) involved in unusual peptide modifications
Abstract
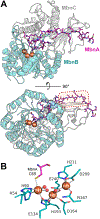
Multinuclear non-heme iron dependent oxidative enzymes (MNIOs), formerly known as domain of unknown function 692 (DUF692), are involved in the post-translational modification of peptides during the biosynthesis of peptide-based natural products. These enzymes catalyze highly unusual and diverse chemical modifications. Several class-defining features of this large family (>14 000 members) are beginning to emerge. Structurally, the enzymes are characterized by a TIM-barrel fold and a set of conserved residues for a di- or tri-iron binding site. They use molecular oxygen to modify peptide substrates, often in a four-electron oxidation taking place at a cysteine residue. This review summarizes the current understanding of MNIOs. Four modifications are discussed in detail: oxazolone-thioamide formation, β-carbon excision, hydantoin-macrocycle formation, and 5-thiooxazole formation. Briefly discussed are two other reactions that do not take place on Cys residues.
Keywords: Dinuclear/trinuclear iron enzyme; Metalloenzyme; Metallophore; Natural product biosynthesis; RiPPs.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
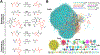

References
-
- Rajakovich LJ, Zhang B, McBride MJ, Boal AK, Krebs C, Bollinger JM Jr.: Emerging Structural and Functional Diversity in Proteins with Dioxygen-Reactive Dinuclear Transition Metal Cofactors. In: Comprehensive Natural Products III: Chemistry and Biology. Liu H-w, Begley TP (Eds), Elsevier, Amsterdam: (2020):215–250.
-
- Ushimaru R, Abe I: Unusual dioxygen-dependent reactions catalyzed by nonheme iron enzymes in natural product biosynthesis. ACS Catal (2023) 13(2):1045–1076.
-
- Feig AL, Lippard SJ: Reactions of non-heme iron(II) centers with dioxygen in biology and chemistry. Chem Rev (1994) 94(3):759–805.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

