Sialic acid blockade inhibits the metastatic spread of prostate cancer to bone
- PMID: 38772281
- PMCID: PMC11134892
- DOI: 10.1016/j.ebiom.2024.105163
Sialic acid blockade inhibits the metastatic spread of prostate cancer to bone
Abstract
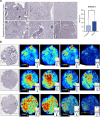
Background: Bone metastasis is a common consequence of advanced prostate cancer. Bisphosphonates can be used to manage symptoms, but there are currently no curative treatments available. Altered tumour cell glycosylation is a hallmark of cancer and is an important driver of a malignant phenotype. In prostate cancer, the sialyltransferase ST6GAL1 is upregulated, and studies show ST6GAL1-mediated aberrant sialylation of N-glycans promotes prostate tumour growth and disease progression.
Methods: Here, we monitor ST6GAL1 in tumour and serum samples from men with aggressive prostate cancer and using in vitro and in vivo models we investigate the role of ST6GAL1 in prostate cancer bone metastasis.
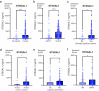
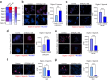
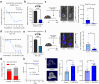
Findings: ST6GAL1 is upregulated in patients with prostate cancer with tumours that have spread to the bone and can promote prostate cancer bone metastasis in vivo. The mechanisms involved are multi-faceted and involve modification of the pre-metastatic niche towards bone resorption to promote the vicious cycle, promoting the development of M2 like macrophages, and the regulation of immunosuppressive sialoglycans. Furthermore, using syngeneic mouse models, we show that inhibiting sialylation can block the spread of prostate tumours to bone.
Interpretation: Our study identifies an important role for ST6GAL1 and α2-6 sialylated N-glycans in prostate cancer bone metastasis, provides proof-of-concept data to show that inhibiting sialylation can suppress the spread of prostate tumours to bone, and highlights sialic acid blockade as an exciting new strategy to develop new therapies for patients with advanced prostate cancer.
Funding: Prostate Cancer Research and the Mark Foundation For Cancer Research, the Medical Research Council and Prostate Cancer UK.
Keywords: Bone metastasis; Glycans; Prostate cancer; Sialic acid; Sialylation; Therapeutics.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests JM and ES are shareholders of GlycoScoreDx Ltd and have filed patent applications related to this work (GB patent numbers GB2,594,103, GB2,595,425 and US patent application 17/780,508). J.F.A.P., N.E., E.R. are shareholders of and employed by GlycoTherapeutics B.V.; C.B. and T.J.B. are shareholders of and scientific advisors of GlycoTherapeutics B.V.; S.J.M is a shareholder of and employed by Synvenio B.V.; J.F.A.P.; and T.J.B. are shareholders of Synvenio B.V.; Radboud University and Radboudumc have filed patent applications related to P-SiaFNEtoc (including patent number 11,639,364). MM has filed patents related to Siglec inhibitors (US patent applications 63/421,007 and 63/497,540). All other authors declare that there are no potential competing interests.
Figures


References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. - PubMed
-
- Kang J., La Manna F., Bonollo F., et al. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett. 2022;530:156–169. - PubMed
-
- Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

