Clinical Trial Protocol for Porcine Islet Xenotransplantation in South Korea
- PMID: 38772544
- PMCID: PMC11621658
- DOI: 10.4093/dmj.2023.0260
Clinical Trial Protocol for Porcine Islet Xenotransplantation in South Korea
Abstract
Backgruound: Islet transplantation holds promise for treating selected type 1 diabetes mellitus patients, yet the scarcity of human donor organs impedes widespread adoption. Porcine islets, deemed a viable alternative, recently demonstrated successful longterm survival without zoonotic risks in a clinically relevant pig-to-non-human primate islet transplantation model. This success prompted the development of a clinical trial protocol for porcine islet xenotransplantation in humans.
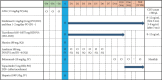
Methods: A single-center, open-label clinical trial initiated by the sponsor will assess the safety and efficacy of porcine islet transplantation for diabetes patients at Gachon Hospital. The protocol received approval from the Gachon Hospital Institutional Review Board (IRB) and the Korean Ministry of Food and Drug Safety (MFDS) under the Investigational New Drug (IND) process. Two diabetic patients, experiencing inadequate glycemic control despite intensive insulin treatment and frequent hypoglycemic unawareness, will be enrolled. Participants and their family members will engage in deliberation before xenotransplantation during the screening period. Each patient will receive islets isolated from designated pathogen-free pigs. Immunosuppressants and systemic infection prophylaxis will follow the program schedule. The primary endpoint is to confirm the safety of porcine islets in patients, and the secondary endpoint is to assess whether porcine islets can reduce insulin dose and the frequency of hypoglycemic unawareness.
Conclusion: A clinical trial protocol adhering to global consensus guidelines for porcine islet xenotransplantation is presented, facilitating streamlined implementation of comparable human trials worldwide.
Keywords: Clinical trial; Diabetes mellitus; Islets of Langerhans; Swine; Transplantation.
Conflict of interest statement
The authors from Tascom, Co. Ltd. and GenNBio have no conflicts of interest.
Figures



References
-
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–90. - PubMed
-
- The Diabetes Control and Complications Trial Research Group Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271–86. - PubMed
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. - PubMed
-
- Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–83. - PubMed
-
- Hering BJ, Cooper DK, Cozzi E, Schuurman HJ, Korbutt GS, Denner J, et al. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes: executive summary. Xenotransplantation. 2009;16:196–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

