Sequence of androgen receptor-targeted vaccination with androgen deprivation therapy affects anti-prostate tumor efficacy
- PMID: 38772685
- PMCID: PMC11110578
- DOI: 10.1136/jitc-2024-008848
Sequence of androgen receptor-targeted vaccination with androgen deprivation therapy affects anti-prostate tumor efficacy
Abstract
Rationale: Androgen deprivation therapy (ADT) is the primary treatment for recurrent and metastatic prostate cancer. In addition to direct antitumor effects, ADT has immunomodulatory effects such as promoting T-cell infiltration and enhancing antigen processing/presentation. Previous studies in our laboratory have demonstrated that ADT also leads to increased expression of the androgen receptor (AR) and increased recognition of prostate tumor cells by AR-specific CD8+T cells. We have also demonstrated that ADT combined with a DNA vaccine encoding the AR significantly slowed tumor growth and improved the survival of prostate tumor-bearing mice. The current study aimed to investigate the impact of the timing and sequencing of ADT with vaccination on the tumor immune microenvironment in murine prostate cancer models to further increase the antitumor efficacy of vaccines.
Methods: Male FVB mice implanted with Myc-CaP tumor cells, or male C57BL/6 mice implanted with TRAMP-C1 prostate tumor cells, were treated with a DNA vaccine encoding AR (pTVG-AR) and ADT. The sequence of administration was evaluated for its effect on tumor growth, and tumor-infiltrating immune populations were characterized.
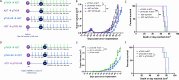
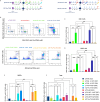
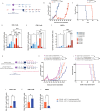
Results: Vaccination prior to ADT (pTVG-AR → ADT) significantly enhanced antitumor responses and survival. This was associated with increased tumor infiltration by CD4+ and CD8+ T cells, including AR-specific CD8+T cells. Depletion of CD8+T cells prior to ADT significantly worsened overall survival. Following ADT treatment, however, Gr1+ myeloid-derived suppressor cells (MDSCs) increased, and this was associated with fewer infiltrating T cells and reduced tumor growth. Inhibiting Gr1+MDSCs recruitment, either by using a CXCR2 antagonist or by cycling androgen deprivation with testosterone replacement, improved antitumor responses and overall survival.
Conclusion: Vaccination prior to ADT significantly improved antitumor responses, mediated in part by increased infiltration of CD8+T cells following ADT. Targeting MDSC recruitment following ADT further enhanced antitumor responses. These findings suggest logical directions for future clinical trials to improve the efficacy of prostate cancer vaccines.
Keywords: Immunotherapy; Myeloid-derived suppressor cell - MDSC; Prostate Cancer; Tumor Microenvironment; Vaccine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DGM has an ownership interest, has received research support and serves as a consultant to Madison Vaccines, which has licensed material described in this manuscript. None of the other authors have relevant potential conflicts of interest.
Figures




Similar articles
-
Myeloid-derived suppressor cells attenuate the antitumor efficacy of radiopharmaceutical therapy using 90Y-NM600 in combination with androgen deprivation therapy in murine prostate tumors.J Immunother Cancer. 2024 Apr 24;12(4):e008760. doi: 10.1136/jitc-2023-008760. J Immunother Cancer. 2024. PMID: 38663936 Free PMC article.
-
Prostate Cancer Cells Express More Androgen Receptor (AR) Following Androgen Deprivation, Improving Recognition by AR-Specific T Cells.Cancer Immunol Res. 2017 Dec;5(12):1074-1085. doi: 10.1158/2326-6066.CIR-16-0390. Epub 2017 Oct 19. Cancer Immunol Res. 2017. PMID: 29051161 Free PMC article.
-
T-Cell Infiltration and Adaptive Treg Resistance in Response to Androgen Deprivation With or Without Vaccination in Localized Prostate Cancer.Clin Cancer Res. 2020 Jul 1;26(13):3182-3192. doi: 10.1158/1078-0432.CCR-19-3372. Epub 2020 Mar 15. Clin Cancer Res. 2020. PMID: 32173650 Free PMC article.
-
Efficacy Against Human Prostate Cancer by Prostate-specific Membrane Antigen-specific, Transforming Growth Factor-β Insensitive Genetically Targeted CD8+ T-cells Derived from Patients with Metastatic Castrate-resistant Disease.Eur Urol. 2018 May;73(5):648-652. doi: 10.1016/j.eururo.2017.12.008. Epub 2017 Dec 21. Eur Urol. 2018. PMID: 29275833 Free PMC article. Review.
-
Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails.Oncogene. 2010 Jun 24;29(25):3593-604. doi: 10.1038/onc.2010.121. Epub 2010 May 3. Oncogene. 2010. PMID: 20440270 Review.
Cited by
-
Immunotherapy in Prostate Cancer: From a "Cold" Tumor to a "Hot" Prospect.Cancers (Basel). 2025 Mar 21;17(7):1064. doi: 10.3390/cancers17071064. Cancers (Basel). 2025. PMID: 40227610 Free PMC article. Review.
-
Sex bias in tumor immunity: insights from immune cells.Theranostics. 2025 Mar 31;15(11):5045-5072. doi: 10.7150/thno.106465. eCollection 2025. Theranostics. 2025. PMID: 40303343 Free PMC article. Review.
-
Transcription factors and hormone receptors: Sex‑specific targets for cancer therapy (Review).Oncol Lett. 2024 Dec 6;29(2):93. doi: 10.3892/ol.2024.14839. eCollection 2025 Feb. Oncol Lett. 2024. PMID: 39691589 Free PMC article. Review.
-
Recent Advances in the Development and Efficacy of Anti-Cancer Vaccines-A Narrative Review.Vaccines (Basel). 2025 Feb 25;13(3):237. doi: 10.3390/vaccines13030237. Vaccines (Basel). 2025. PMID: 40266115 Free PMC article. Review.
References
-
- Huggins C. Studies on prostatic cancer. Arch Surg 1941;43:209. 10.1001/archsurg.1941.01210140043004 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
