Seipin deficiency-induced lipid dysregulation leads to hypomyelination-associated cognitive deficits via compromising oligodendrocyte precursor cell differentiation
- PMID: 38773070
- PMCID: PMC11109229
- DOI: 10.1038/s41419-024-06737-z
Seipin deficiency-induced lipid dysregulation leads to hypomyelination-associated cognitive deficits via compromising oligodendrocyte precursor cell differentiation
Abstract
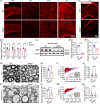
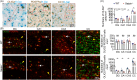
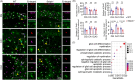
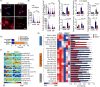
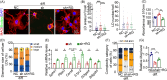
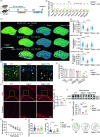
Seipin is one key mediator of lipid metabolism that is highly expressed in adipose tissues as well as in the brain. Lack of Seipin gene, Bscl2, leads to not only severe lipid metabolic disorders but also cognitive impairments and motor disabilities. Myelin, composed mainly of lipids, facilitates nerve transmission and is important for motor coordination and learning. Whether Seipin deficiency-leaded defects in learning and motor coordination is underlined by lipid dysregulation and its consequent myelin abnormalities remains to be elucidated. In the present study, we verified the expression of Seipin in oligodendrocytes (OLs) and their precursors, oligodendrocyte precursor cells (OPCs), and demonstrated that Seipin deficiency compromised OPC differentiation, which led to decreased OL numbers, myelin protein, myelinated fiber proportion and thickness of myelin. Deficiency of Seipin resulted in impaired spatial cognition and motor coordination in mice. Mechanistically, Seipin deficiency suppressed sphingolipid metabolism-related genes in OPCs and caused morphological abnormalities in lipid droplets (LDs), which markedly impeded OPC differentiation. Importantly, rosiglitazone, one agonist of PPAR-gamma, substantially restored phenotypes resulting from Seipin deficiency, such as aberrant LDs, reduced sphingolipids, obstructed OPC differentiation, and neurobehavioral defects. Collectively, the present study elucidated how Seipin deficiency-induced lipid dysregulation leads to neurobehavioral deficits via impairing myelination, which may pave the way for developing novel intervention strategy for treating metabolism-involved neurological disorders.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Seipin Deficiency Impairs Motor Coordination in Mice by Compromising Spinal Cord Myelination.Neuromolecular Med. 2025 Jan 27;27(1):12. doi: 10.1007/s12017-025-08834-4. Neuromolecular Med. 2025. PMID: 39869141
-
C1ql1 expression in oligodendrocyte progenitor cells promotes oligodendrocyte differentiation.FEBS J. 2025 Jan;292(1):52-74. doi: 10.1111/febs.17256. Epub 2024 Sep 11. FEBS J. 2025. PMID: 39257292
-
Oligodendrocyte progenitor cells' fate after neonatal asphyxia-Puzzling implications for the development of hypoxic-ischemic encephalopathy.Brain Pathol. 2024 Nov;34(6):e13255. doi: 10.1111/bpa.13255. Epub 2024 Mar 19. Brain Pathol. 2024. PMID: 38504469 Free PMC article.
-
Impact of Sex Differences in Oligodendrocytes and Their Progenitor Cells on the Pathophysiology of Neuropsychiatric Disorders.J Nippon Med Sch. 2025;92(3):226-233. doi: 10.1272/jnms.JNMS.2025_92-306. J Nippon Med Sch. 2025. PMID: 40603012 Review.
-
Role of growth factors and their interplay during oligodendroglial differentiation and maturation.Cytokine Growth Factor Rev. 2025 Aug;84:47-58. doi: 10.1016/j.cytogfr.2025.07.002. Epub 2025 Jul 10. Cytokine Growth Factor Rev. 2025. PMID: 40707311 Review.
Cited by
-
Direct Current Stimulation (DCS) Modulates Lipid Metabolism and Intercellular Vesicular Trafficking in SHSY-5Y Cell Line: Implications for Parkinson's Disease.J Neurochem. 2025 Feb;169(2):e70014. doi: 10.1111/jnc.70014. J Neurochem. 2025. PMID: 39930930 Free PMC article.
-
Lipid metabolism in health and disease: Mechanistic and therapeutic insights for Parkinson's disease.Chin Med J (Engl). 2025 Jun 20;138(12):1411-1423. doi: 10.1097/CM9.0000000000003627. Epub 2025 May 26. Chin Med J (Engl). 2025. PMID: 40419446 Free PMC article. Review.
-
Long-read RNA-sequencing reveals transcript-specific regulation in human-derived cortical neurons.Open Biol. 2025 Jul;15(7):250200. doi: 10.1098/rsob.250200. Epub 2025 Jul 30. Open Biol. 2025. PMID: 40735840 Free PMC article.
References
-
- Zhu J, Lam SM, Yang L, Liang J, Ding M, Shui G, et al. Reduced phosphatidylcholine synthesis suppresses the embryonic lethality of seipin deficiency. Life Metab. 2022;1:175–89. doi: 10.1093/lifemeta/loac021. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

