Determinants of gastric cancer immune escape identified from non-coding immune-landscape quantitative trait loci
- PMID: 38773080
- PMCID: PMC11109163
- DOI: 10.1038/s41467-024-48436-5
Determinants of gastric cancer immune escape identified from non-coding immune-landscape quantitative trait loci
Abstract
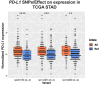
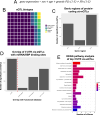
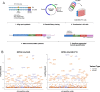
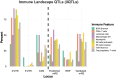
The landscape of non-coding mutations in cancer progression and immune evasion is largely unexplored. Here, we identify transcrptome-wide somatic and germline 3' untranslated region (3'-UTR) variants from 375 gastric cancer patients from The Cancer Genome Atlas. By performing gene expression quantitative trait loci (eQTL) and immune landscape QTL (ilQTL) analysis, we discover 3'-UTR variants with cis effects on expression and immune landscape phenotypes, such as immune cell infiltration and T cell receptor diversity. Using a massively parallel reporter assay, we distinguish between causal and correlative effects of 3'-UTR eQTLs in immune-related genes. Our approach identifies numerous 3'-UTR eQTLs and ilQTLs, providing a unique resource for the identification of immunotherapeutic targets and biomarkers. A prioritized ilQTL variant signature predicts response to immunotherapy better than standard-of-care PD-L1 expression in independent patient cohorts, showcasing the untapped potential of non-coding mutations in cancer.
© 2024. The Author(s).
Conflict of interest statement
I.S.V. consults for Guidepoint Global, Cowen, Mosaic, and NextRNA. Beth Israel Deaconess Medical Center has filed a patent application based on this work for “Methods and compositions related to non-coding variants for the prediction of response to cancer immunotherapy” under 63/378,392, where I.S.V., F.J.S., C.M., and E.K. are named as co-inventors. The remaining authors declare no competing interests.
Figures

Similar articles
-
Aberrant PD-L1 expression through 3'-UTR disruption in multiple cancers.Nature. 2016 Jun 16;534(7607):402-6. doi: 10.1038/nature18294. Epub 2016 May 23. Nature. 2016. PMID: 27281199
-
Overexpression of DAPK1-mediated inhibition of IKKβ/CSN5/PD-L1 axis enhances natural killer cell killing ability and inhibits tumor immune evasion in gastric cancer.Cell Immunol. 2022 Feb;372:104469. doi: 10.1016/j.cellimm.2021.104469. Epub 2021 Dec 10. Cell Immunol. 2022. PMID: 35114597
-
miR-105-5p regulates PD-L1 expression and tumor immunogenicity in gastric cancer.Cancer Lett. 2021 Oct 10;518:115-126. doi: 10.1016/j.canlet.2021.05.037. Epub 2021 Jun 23. Cancer Lett. 2021. PMID: 34098061 Free PMC article.
-
Immunotherapy for Esophageal and Gastric Cancer.Am Soc Clin Oncol Educ Book. 2017;37:292-300. doi: 10.1200/EDBK_175231. Am Soc Clin Oncol Educ Book. 2017. PMID: 28561677 Review.
-
Progress and prospects of immune checkpoint inhibitors in advanced gastric cancer.Future Oncol. 2021 Apr;17(12):1553-1569. doi: 10.2217/fon-2020-0829. Epub 2021 Jan 5. Future Oncol. 2021. PMID: 33397136 Review.
Cited by
-
Mechanisms of isorhamnetin inhibition of osteoclast differentiation: insights from molecular dynamics simulations and in vitro/in vivo experiments.Front Pharmacol. 2025 Apr 28;16:1551257. doi: 10.3389/fphar.2025.1551257. eCollection 2025. Front Pharmacol. 2025. PMID: 40356982 Free PMC article.
-
High-resolution subtyping of fibroblasts in gastric cancer reveals diversity among fibroblast subsets and an association between the MFAP5-fibroblast subset and immunotherapy.Front Immunol. 2024 Oct 25;15:1446613. doi: 10.3389/fimmu.2024.1446613. eCollection 2024. Front Immunol. 2024. PMID: 39524442 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

