Explainable chemical artificial intelligence from accurate machine learning of real-space chemical descriptors
- PMID: 38773090
- PMCID: PMC11522690
- DOI: 10.1038/s41467-024-48567-9
Explainable chemical artificial intelligence from accurate machine learning of real-space chemical descriptors
Abstract
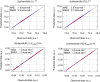
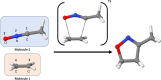
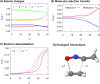
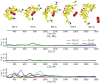
Machine-learned computational chemistry has led to a paradoxical situation in which molecular properties can be accurately predicted, but they are difficult to interpret. Explainable AI (XAI) tools can be used to analyze complex models, but they are highly dependent on the AI technique and the origin of the reference data. Alternatively, interpretable real-space tools can be employed directly, but they are often expensive to compute. To address this dilemma between explainability and accuracy, we developed SchNet4AIM, a SchNet-based architecture capable of dealing with local one-body (atomic) and two-body (interatomic) descriptors. The performance of SchNet4AIM is tested by predicting a wide collection of real-space quantities ranging from atomic charges and delocalization indices to pairwise interaction energies. The accuracy and speed of SchNet4AIM breaks the bottleneck that has prevented the use of real-space chemical descriptors in complex systems. We show that the group delocalization indices, arising from our physically rigorous atomistic predictions, provide reliable indicators of supramolecular binding events, thus contributing to the development of Explainable Chemical Artificial Intelligence (XCAI) models.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Explanatory pragmatism: a context-sensitive framework for explainable medical AI.Ethics Inf Technol. 2022;24(1):13. doi: 10.1007/s10676-022-09632-3. Epub 2022 Feb 28. Ethics Inf Technol. 2022. PMID: 35250370 Free PMC article.
-
Explainable AI: Machine Learning Interpretation in Blackcurrant Powders.Sensors (Basel). 2024 May 17;24(10):3198. doi: 10.3390/s24103198. Sensors (Basel). 2024. PMID: 38794052 Free PMC article.
-
Model-agnostic explainable artificial intelligence tools for severity prediction and symptom analysis on Indian COVID-19 data.Front Artif Intell. 2023 Dec 4;6:1272506. doi: 10.3389/frai.2023.1272506. eCollection 2023. Front Artif Intell. 2023. PMID: 38111787 Free PMC article.
-
Explainable AI for Bioinformatics: Methods, Tools and Applications.Brief Bioinform. 2023 Sep 20;24(5):bbad236. doi: 10.1093/bib/bbad236. Brief Bioinform. 2023. PMID: 37478371 Review.
-
Explainability and white box in drug discovery.Chem Biol Drug Des. 2023 Jul;102(1):217-233. doi: 10.1111/cbdd.14262. Epub 2023 Apr 27. Chem Biol Drug Des. 2023. PMID: 37105727 Review.
Cited by
-
Quantum Topological Atomic Properties of 44K molecules.Sci Data. 2024 Aug 29;11(1):945. doi: 10.1038/s41597-024-03723-0. Sci Data. 2024. PMID: 39209874 Free PMC article.
-
"Amide - amine + alcohol = carboxylic acid." chemical reactions as linear algebraic analogies in graph neural networks.Chem Sci. 2025 Apr 23;16(24):10895-10908. doi: 10.1039/d4sc05655h. eCollection 2025 Jun 18. Chem Sci. 2025. PMID: 40395375 Free PMC article.
-
Consensus Modeling Strategies for Predicting Transthyretin Binding Affinity from Tox24 Challenge Data.Chem Res Toxicol. 2025 Jun 16;38(6):1061-1071. doi: 10.1021/acs.chemrestox.5c00018. Epub 2025 May 15. Chem Res Toxicol. 2025. PMID: 40371923 Free PMC article.
-
An explainable "family bucket" model for simultaneous prediction of K-edge XANES for multiple light transition metals.Chem Sci. 2025 Aug 1;16(34):15571-15586. doi: 10.1039/d5sc00494b. eCollection 2025 Aug 27. Chem Sci. 2025. PMID: 40756976 Free PMC article.
-
Organic Fusion of Molecular Simulation and Wet-Lab Validation: A Promising High-Throughput Strategy for Screening Bioactive Food Peptides.Foods. 2025 Aug 20;14(16):2890. doi: 10.3390/foods14162890. Foods. 2025. PMID: 40870802 Free PMC article. Review.
References
-
- Singh, V., Patra, S., Murugan, N. A., Toncu, D.-C. & Tiwari, A. Recent trends in computational tools and data-driven modeling for advanced materials. Mater. Adv.3, 4069–4087 (2022).
-
- Cembran, A., Bernardi, F., Olivucci, M. & Garavelli, M. Counterion controlled photoisomerization of retinal chromophore models: a computational investigation. J. Am. Chem. Soc.126, 16018–16037 (2004). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

