Ferritin nanocage-enabled detection of pathological tau in living human retinal cells
- PMID: 38773170
- PMCID: PMC11109090
- DOI: 10.1038/s41598-024-62188-8
Ferritin nanocage-enabled detection of pathological tau in living human retinal cells
Abstract
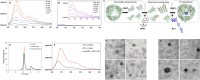
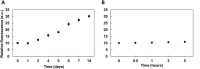
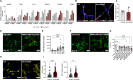
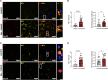
Tauopathies, including Alzheimer's disease and Frontotemporal Dementia, are debilitating neurodegenerative disorders marked by cognitive decline. Despite extensive research, achieving effective treatments and significant symptom management remains challenging. Accurate diagnosis is crucial for developing effective therapeutic strategies, with hyperphosphorylated protein units and tau oligomers serving as reliable biomarkers for these conditions. This study introduces a novel approach using nanotechnology to enhance the diagnostic process for tauopathies. We developed humanized ferritin nanocages, a novel nanoscale delivery system, designed to encapsulate and transport a tau-specific fluorophore, BT1, into human retinal cells for detecting neurofibrillary tangles in retinal tissue, a key marker of tauopathies. The delivery of BT1 into living cells was successfully achieved through these nanocages, demonstrating efficient encapsulation and delivery into retinal cells derived from human induced pluripotent stem cells. Our experiments confirmed the colocalization of BT1 with pathological forms of tau in living retinal cells, highlighting the method's potential in identifying tauopathies. Using ferritin nanocages for BT1 delivery represents a significant contribution to nanobiotechnology, particularly in neurodegenerative disease diagnostics. This method offers a promising tool for the early detection of tau tangles in retinal tissue, with significant implications for improving the diagnosis and management of tauopathies. This study exemplifies the integration of nanotechnology with biomedical science, expanding the frontiers of nanomedicine and diagnostic techniques.
Keywords: Alzheimer’s disease; BODYPY tau fluorophores; Diagnostic probes; Ferritin nanocages; Frontotemporal Dementia; Induced pluripotent stem cells; Nanobiotechnology; Nanomedicine; Nanoscale delivery systems; Neurodegenerative diseases; Retinal tissue detection; Tauopathies.
© 2024. The Author(s).
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. YG is employed by D-Tails s.r.l.; MP and SG were employed by D-Tails s.r.l; SDA and AB are scientific advisors of D-Tails s.r.l. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Tau oligomers as potential targets for early diagnosis of tauopathy.J Alzheimers Dis. 2014;40 Suppl 1:S91-6. doi: 10.3233/JAD-132429. J Alzheimers Dis. 2014. PMID: 24595194
-
Altered Proteostasis in Neurodegenerative Tauopathies.Adv Exp Med Biol. 2020;1233:177-194. doi: 10.1007/978-3-030-38266-7_7. Adv Exp Med Biol. 2020. PMID: 32274757 Review.
-
Mechanisms of tau self-aggregation and neurotoxicity.Curr Alzheimer Res. 2011 Sep;8(6):608-14. doi: 10.2174/156720511796717258. Curr Alzheimer Res. 2011. PMID: 21605046 Review.
-
Tauopathies and tau oligomers.J Alzheimers Dis. 2013;37(3):565-8. doi: 10.3233/JAD-130653. J Alzheimers Dis. 2013. PMID: 23948895 Review.
-
Tau phosphorylation and aggregation as a therapeutic target in tauopathies.CNS Neurol Disord Drug Targets. 2010 Dec;9(6):727-40. doi: 10.2174/187152710793237403. CNS Neurol Disord Drug Targets. 2010. PMID: 20942789 Review.
Cited by
-
Understanding retinal tau pathology through functional 2D and 3D iPSC-derived in vitro retinal models.Acta Neuropathol Commun. 2025 Jan 29;13(1):19. doi: 10.1186/s40478-024-01920-x. Acta Neuropathol Commun. 2025. PMID: 39881365 Free PMC article.
-
Bezafibrate treatment rescues neurodevelopmental and neurodegenerative defects in 3D cortical organoid model of MAPT frontotemporal dementia.Alzheimers Dement. 2025 Aug;21(8):e70419. doi: 10.1002/alz.70419. Alzheimers Dement. 2025. PMID: 40810332 Free PMC article.
-
Short-chain polyphosphates induce tau fibrillation and neurotoxicity in human iPSC-derived retinal neurons.Cell Death Dis. 2025 May 9;16(1):369. doi: 10.1038/s41419-025-07662-5. Cell Death Dis. 2025. PMID: 40346074 Free PMC article.
References
MeSH terms
Grants and funding
- PRIN 2022 2022CFP7RF/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- FSE 2014-2020 19036AP000000019/Regione Lazio (Region of Lazio)
- FSE 2014-2020 A0112E0073/Regione Lazio (Region of Lazio)
- Synergy grant program, project ASTRA grant agreement No 855923/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- Pathfinder Open Programme, project ivBM-4PAP grant agreement No 101098989/EC | EU Framework Programme for Research and Innovation H2020 | H2020 European Institute of Innovation and Technology (H2020 The European Institute of Innovation and Technology)
LinkOut - more resources
Full Text Sources

