Glycine by enteral route does not improve major clinical outcomes in severe COVID-19: a randomized clinical pilot trial
- PMID: 38773199
- PMCID: PMC11109244
- DOI: 10.1038/s41598-024-62321-7
Glycine by enteral route does not improve major clinical outcomes in severe COVID-19: a randomized clinical pilot trial
Abstract
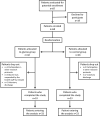
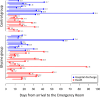
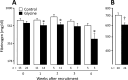
There is a worrying scarcity of drug options for patients with severe COVID-19. Glycine possesses anti-inflammatory, cytoprotective, endothelium-protective, and platelet-antiaggregant properties, so its use in these patients seems promising. In this open label, controlled clinical trial, inpatients with severe COVID-19 requiring mechanical ventilation randomly received usual care (control group) or usual care plus 0.5 g/kg/day glycine by the enteral route (experimental group). Major outcomes included mortality, time to weaning from mechanical ventilation, total time on mechanical ventilation, and time from study recruitment to death. Secondary outcomes included laboratory tests and serum cytokines. Patients from experimental (n = 33) and control groups (n = 23) did not differ in basal characteristics. There were no differences in mortality (glycine group, 63.6% vs control group, 52.2%, p = 0.60) nor in any other major outcome. Glycine intake was associated with lower fibrinogen levels, either evaluated per week of follow-up (p < 0.05 at weeks 1, 2, and 4) or as weighted mean during the whole hospitalization (608.7 ± 17.7 mg/dl vs control 712.2 ± 25.0 mg/dl, p = 0.001), but did not modify any other laboratory test or cytokine concentration. In summary, in severe COVID-19 glycine was unable to modify major clinical outcomes, serum cytokines or most laboratory tests, but was associated with lower serum fibrinogen concentration.Registration: ClinicalTrials.gov NCT04443673, 23/06/2020.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Randomised controlled trial comparing efficacy and safety of high versus low Low-Molecular Weight Heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol.Trials. 2020 Jun 26;21(1):574. doi: 10.1186/s13063-020-04475-z. Trials. 2020. PMID: 32586394 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Coagulopathy of hospitalised COVID-19: A Pragmatic Randomised Controlled Trial of Therapeutic Anticoagulation versus Standard Care as a Rapid Response to the COVID-19 Pandemic (RAPID COVID COAG - RAPID Trial): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Mar 10;22(1):202. doi: 10.1186/s13063-021-05076-0. Trials. 2021. PMID: 33691765 Free PMC article.
-
Recombinant human C1 esterase inhibitor (conestat alfa) in the prevention of severe SARS-CoV-2 infection in hospitalized patients with COVID-19: A structured summary of a study protocol for a randomized, parallel-group, open-label, multi-center pilot trial (PROTECT-COVID-19).Trials. 2021 Jan 4;22(1):1. doi: 10.1186/s13063-020-04976-x. Trials. 2021. PMID: 33397449 Free PMC article.
Cited by
-
Lower Serum Magnesium Is Associated with Mortality in Severe COVID-19: A Secondary Analysis of a Randomized Trial.Biol Trace Elem Res. 2025 Apr 15. doi: 10.1007/s12011-025-04619-9. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40234280
References
-
- World Health Organization. Therapeutics and COVID-19. Living guideline (Accessed 13 January 2023). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

