A recurrent de novo missense mutation in COL1A1 causes osteogenesis imperfecta type II and preterm delivery in Normande cattle
- PMID: 38773368
- PMCID: PMC11107018
- DOI: 10.1186/s12711-024-00909-3
A recurrent de novo missense mutation in COL1A1 causes osteogenesis imperfecta type II and preterm delivery in Normande cattle
Abstract
Background: Nine male and eight female calves born to a Normande artificial insemination bull named "Ly" were referred to the French National Observatory of Bovine Abnormalities for multiple fractures, shortened gestation, and stillbirth or perinatal mortality.
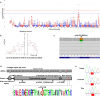
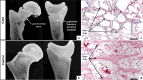
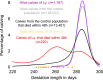
Results: Using Illumina BovineSNP50 array genotypes from affected calves and 84 half-sib controls, the associated locus was mapped to a 6.5-Mb interval on chromosome 19, assuming autosomal inheritance with germline mosaicism. Subsequent comparison of the whole-genome sequences of one case and 5116 control genomes, followed by genotyping in the affected pedigree, identified a de novo missense substitution within the NC1 domain of the COL1A1 gene (Chr19 g.36,473,965G > A; p.D1412N) as unique candidate variant. Interestingly, the affected residue was completely conserved among 243 vertebrate orthologs, and the same substitution in humans has been reported to cause type II osteogenesis imperfecta (OI), a connective tissue disorder that is characterized primarily by bone deformity and fragility. Moreover, three COL1A1 mutations have been described to cause the same syndrome in cattle. Necropsy, computed tomography, radiology, and histology confirmed the diagnosis of type II OI, further supporting the causality of this variant. In addition, a detailed analysis of gestation length and perinatal mortality in 1387 offspring of Ly and more than 160,000 progeny of 63 control bulls allowed us to statistically confirm in a large pedigree the association between type II OI and preterm delivery, which is probably due to premature rupture of fetal membranes and has been reported in several isolated cases of type II OI in humans and cattle. Finally, analysis of perinatal mortality rates and segregation distortion supported a low level of germ cell mosaicism in Ly, with an estimate of 4.5% to 7.7% of mutant sperm and thus 63 to 107 affected calves born. These numbers contrast with the 17 cases reported and raise concerns about the underreporting of congenital defects to heredo-surveillance platforms, even for textbook genetic syndromes.
Conclusions: In conclusion, we describe a large animal model for a recurrent substitution in COL1A1 that is responsible for type II OI in humans. More generally, this study highlights the utility of such datasets and large half-sib families available in livestock species to characterize sporadic genetic defects.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

