Seroprevalence and placental transfer of SARS-CoV-2 antibodies in unvaccinated pregnant women
- PMID: 38773493
- PMCID: PMC11110414
- DOI: 10.1186/s12879-024-09399-6
Seroprevalence and placental transfer of SARS-CoV-2 antibodies in unvaccinated pregnant women
Abstract
Purpose: Pregnant women are at risk of severe SARS-CoV-2 infection, potentially leading to obstetric and neonatal complications. Placental transfer of antibodies directed to SARS-CoV-2 may be protective against neonatal COVID-19, but this remains to be studied. We aimed to determine the seroprevalence of SARS-CoV-2 antibodies in a population of unvaccinated pregnant women and to determine the placental transfer of these antibodies.
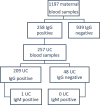
Methodology: A total of 1197 unvaccinated women with mostly unknown pre-study SARS-CoV-2 infection status, were tested at delivery for SARS-CoV-2 spike protein IgG antibodies during the first year of the pandemic. Umbilical cord samples were collected and assessed for seropositivity if the mother was seropositive. Maternal characteristics, pregnancy and neonatal outcomes and data on SARS-CoV-2 infection were extracted from medical records.
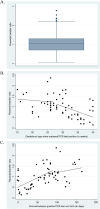
Results: Specific IgG were detected in 258 women (21.6%). A significant placental transfer to the newborn was observed in 81.3% of cases. The earlier in the 2nd and 3rd trimesters that the mother had contracted the disease and the more symptomatic she was, the greater the likelihood of transplacental transfer of IgG to her newborn.
Conclusion: Approximately one in five women had detectable anti-SARS-CoV-2 spike protein IgG antibodies at delivery during the first year of the pandemic, and these antibodies were significantly transferred to their fetuses. This research provides further evidence to better understand the dynamics of the placental transfer of SARS-CoV-2 IgG antibodies from mothers to their newborns, which is necessary to improve vaccination strategies.
Keywords: Antibody; COVID-19; Placental transfer; Pregnancy; SARS-COV-2 antibodies; Seroprevalence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Vousden N, Bunch K, Morris E, Simpson N, Gale C, O’Brien P, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS). Farrar D, editor. PLoS One. 2021;16(5):e0251123. doi: 10.1371/journal.pone.0251123. - DOI - PMC - PubMed
-
- Favre G, Maisonneuve E, Pomar L, Daire C, Poncelet C, Quibel T, et al. Maternal and perinatal outcomes following pre-Delta, Delta, and Omicron SARS-CoV-2 variants infection among unvaccinated pregnant women in France and Switzerland: a prospective cohort study using the COVI-PREG registry. Lancet Reg Heal - Eur. 2023;26:100569. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2666776222002654. - PMC - PubMed
-
- de Bruin O, Engjom H, Vousden N, Ramakrishnan R, Aabakke AJM, Äyräs O, et al. Variations across Europe in hospitalization and management of pregnant women with <scp>SARS‐CoV</scp> ‐2 during the initial phase of the pandemic: Multi‐national population‐based cohort study using the International Network of Obstetric Survey Systems ( <. Acta Obstet Gynecol Scand. 2023; Available from: 10.1111/aogs.14643. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

