Experimental colitis in young Tg2576 mice accelerates the onset of an Alzheimer's-like clinical phenotype
- PMID: 38773640
- PMCID: PMC11110243
- DOI: 10.1186/s13195-024-01471-2
Experimental colitis in young Tg2576 mice accelerates the onset of an Alzheimer's-like clinical phenotype
Abstract
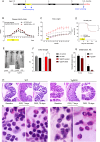
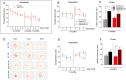
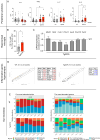
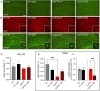
Systemic inflammation and neuroinflammation affect the natural course of the sporadic form of Alzheimer's disease (AD), as supported by epidemiological and preclinical data, and several epidemiological studies indicate a higher prevalence of AD in patients with inflammatory bowel disease. In this study, we explored whether colitis induced by dextran sulfate sodium (DSS) in young, presymptomatic/preplaque mice worsens and/or anticipates age-dependent cognitive impairment in Tg2576, a widely used mouse model of AD. We demonstrated that DSS colitis induced in young Tg2576 mice anticipates the onset age of learning and memory deficit in the Morris water maze test. To explore potential mechanisms behind the acceleration of cognitive decline in Tg2576 mice by DSS colitis, we focused on gut microbiota, systemic inflammation and neuroinflammation markers. We observed a Firmicutes/Bacteroidetes ratio change in Tg2576 DSS animals comparable to that of elderly Tg2576 mice, suggesting accelerated microbiota aging in Tg2576 DSS mice, a change not observed in C57BL6 DSS mice. We also observed substantial differences between Tg2576 and WT mice in several inflammation and neuroinflammation-related parameters as early as 3 months of age, well before plaque deposition, a picture which evolved rapidly (between 3 and 5.5 months of age) in contrast to Tg2576 and WT littermates not treated with DSS. In detail, following induction of DSS colitis, WT and Tg2576 mice exhibited contrasting features in the expression level of inflammation-evoked astrocyte-associated genes in the hippocampus. No changes in microglial features occurred in the hippocampus between the experimental groups, whereas a reduced glial fibrillary acidic protein immunoreactivity was observed in Tg2576 vs. WT mice. This finding may reflect an atrophic, "loss-of-function" profile, further exacerbated by DSS where a decreased of GFAP mRNA expression level was detected. In conclusion, we suggest that as-yet unidentified peripheral mediators evoked by DSS colitis and involving the gut-brain axis emphasize an astrocyte "loss-of-function" profile present in young Tg2576 mice, leading to impaired synaptic morphological and functional integrity as a very early sign of AD.
Keywords: Astrocytes; Cytokines/chemokines; Gut microbiota/microbiome; Neuroinflammation; Preclinical Alzheimer’s disease; Systemic inflammation; Tg2576 mice.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

