In vivo fluid dynamics of the Ventura interatrial shunt device in patients with heart failure
- PMID: 38773938
- PMCID: PMC11424381
- DOI: 10.1002/ehf2.14859
In vivo fluid dynamics of the Ventura interatrial shunt device in patients with heart failure
Abstract
Aims: Interatrial shunts are under evaluation as a treatment for heart failure (HF); however, their in vivo flow performance has not been quantitatively studied. We aimed to investigate the fluid dynamics properties of the 0.51 cm orifice diameter Ventura shunt and assess its lumen integrity with serial transesophageal echocardiography (TEE).
Methods and results: Computational fluid dynamics (CFD) and bench flow tests were used to establish the flow-pressure relationship of the shunt. Open-label patients from the RELIEVE-HF trial underwent TEE at shunt implant and at 6 and 12 month follow-up. Shunt effective diameter (Deff) was derived from the vena contracta, and flow was determined by the continuity equation. CFD and bench studies independently validated that the shunt's discharge coefficient was 0.88 to 0.89. The device was successfully implanted in all 97 enrolled patients; mean age was 70 ± 11 years, 97% were NYHA class III, and 51% had LVEF ≤40%. Patency was confirmed in all instances, except for one stenotic shunt at 6 months. Deff remained unchanged from baseline at 12 months (0.47 ± 0.01 cm, P = 0.376), as did the trans-shunt mean pressure gradient (5.1 ± 3.9 mmHg, P = 0.316) and flow (1137 ± 463 mL/min, P = 0.384). TEE measured flow versus pressure closely correlated (R2 ≥ 0.98) with a fluid dynamics model. At 12 months, the pulmonary/systemic flow Qp/Qs ratio was 1.22 ± 0.12.
Conclusions: When implanted in patients with advanced HF, this small interatrial shunt demonstrated predictable and durable patency and performance.
Keywords: Flow dynamics; Heart failure; Interatrial shunt; Transesophageal echocardiography.
© 2024 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Drs. Pfeiffer's, Boehmer's, and Gorcsan's employer, Penn State University, receives research fees from V‐Wave supporting the RELIEVE‐HF Echocardiography Core Laboratory. Dr. Pfeiffer has received speaker honoraria for Abbott and Ancora. Dr. Bayes has received speaker honoraria and/or consulting for AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Roche Diagnostics, Vifor. Dr Eigler is an employee and a shareholder of V‐Wave. Dr. Abraham receives personal fees and is a shareholder of V‐Wave. Dr. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, Amgen, Boehringer Ingelheim; has served as a consultant to Abbott, Daiichi Sankyo, Ablative Solutions, CorFlow, Cardiomech, Robocath, Miracor, Vectorious, Apollo Therapeutics, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Elucid Bio, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, Cardiac Success, HighLife; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, Xenter. Dr. Stone's employer, Mount Sinai Hospital, receives research grants from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense‐Webster, Shockwave, Vascular Dynamics, Pulnovo, V‐wave. Dr. Núñez has received speaker honoraria and/or consulting for Alleviant, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, NovoNordisk, Rovi, and Vifor. Dr. Anker receives grants and personal fees from Vifor and Abbott Vascular, and personal fees for consultancies, trial committee work and/or lectures from Actimed, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Bioventrix, Brahms, Cardiac Dimensions, Cardior, Cordio, CVRx, Cytokinetics, Edwards, Farraday Pharmaceuticals, GSK, HeartKinetics, Impulse Dynamics, Novartis, Occlutech, Pfizer, Repairon, Sensible Medical, Servier, Vectorious, and V‐Wave; is a named co‐inventor of two patent applications regarding MR‐proANP (DE 102007010834 & DE 102007022367), but he does not benefit personally from the related issued patents.
Figures

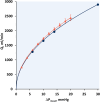
 represents data from CFD simulations in blood and
represents data from CFD simulations in blood and  are bench measurements in saline. Error bars are standard deviations. Curved lines are respective fits to model haemodynamic equation (1) with R
2 > 0.99 for each fit.
, mean pressure gradient; Q, flow.
are bench measurements in saline. Error bars are standard deviations. Curved lines are respective fits to model haemodynamic equation (1) with R
2 > 0.99 for each fit.
, mean pressure gradient; Q, flow.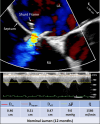


 below this line indicate that the shunt orifice size was artifactually reduced due to non‐coaxial imaging (pseudo stenotic). The red triangle
below this line indicate that the shunt orifice size was artifactually reduced due to non‐coaxial imaging (pseudo stenotic). The red triangle  represents a single patient with a stenotic shunt at 6 month follow‐up. That patient exited the study upon receiving a left ventricular assist device (LVAD) at 8 months at which time the shunt was occluded. Mean ± standard deviation values are exclusive of stenotic shunt.
represents a single patient with a stenotic shunt at 6 month follow‐up. That patient exited the study upon receiving a left ventricular assist device (LVAD) at 8 months at which time the shunt was occluded. Mean ± standard deviation values are exclusive of stenotic shunt.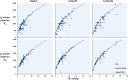
 (blue circles), with population means ± standard deviations (blue crosses with error bars). The red triangle
(blue circles), with population means ± standard deviations (blue crosses with error bars). The red triangle  represents a single patient with a stenotic shunt at 6 month follow‐up. Patient data are compared with the model derived from computational fluid dynamics simulation (black curves).
represents a single patient with a stenotic shunt at 6 month follow‐up. Patient data are compared with the model derived from computational fluid dynamics simulation (black curves).References
-
- Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP‐HF II): a randomised, multicentre, blinded, sham‐controlled trial. Lancet 2022;399:1130‐1140. doi: 10.1016/S0140-6736(22)00016-2 - DOI - PubMed
-
- Shah SJ, Feldman T, Ricciardi MJ, Kahwash R, Lilly S, Litwin S, et al. One‐year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the reduce elevated left atrial pressure in patients with heart failure (REDUCE LAP‐HF I) trial: a randomized clinical trial. JAMA Cardiol 2018;3:968‐977. doi: 10.1001/jamacardio.2018.2936 - DOI - PMC - PubMed
-
- Kaye DM, Hasenfuß G, Neuzil P, Post MC, Doughty R, Trochu JN, et al. One‐year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail 2016;9:e003662. doi: 10.1161/CIRCHEARTFAILURE.116.003662 - DOI - PMC - PubMed
-
- Rodés‐Cabau J, Bernier M, Amat‐Santos IJ, Ben Gal T, Nombela‐Franco L, García del Blanco B, et al. Interatrial shunting for heart failure: early and late results from the first‐in‐human experience with the V‐wave system. JACC Cardiovasc Interv 2018;11:2300‐2310. doi: 10.1016/j.jcin.2018.07.001 - DOI - PubMed
-
- Paitazoglou C, Bergmann MW, Özdemir R, Pfister R, Bartunek J, Kilic T, et al. One‐year results of the first‐in‐man study investigating the atrial flow regulator for left atrial shunting in symptomatic heart failure patients: the PRELIEVE study. Eur J Heart Fail 2021;23:800‐810. doi: 10.1002/ejhf.2119 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

