AXL-specific single domain antibodies show diagnostic potential and anti-tumor activity in Acute Myeloid Leukemia
- PMID: 38773967
- PMCID: PMC11103505
- DOI: 10.7150/thno.91456
AXL-specific single domain antibodies show diagnostic potential and anti-tumor activity in Acute Myeloid Leukemia
Abstract
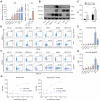
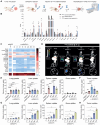
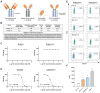
Rationale: AXL expression has been identified as a prognostic factor in acute myeloid leukemia (AML) and is detectable in approximately 50% of AML patients. In this study, we developed AXL-specific single domain antibodies (sdAbs), cross-reactive for both mouse and human AXL protein, to non-invasively image and treat AXL-expressing cancer cells. Methods: AXL-specific sdAbs were induced by immunizing an alpaca with mouse and human AXL proteins. SdAbs were characterized using ELISA, flow cytometry, surface plasmon resonance and the AlphaFold2 software. A lead compound was selected and labeled with 99mTc for evaluation as a diagnostic tool in mouse models of human (THP-1 cells) or mouse (C1498 cells) AML using SPECT/CT imaging. For therapeutic purposes, the lead compound was fused to a mouse IgG2a-Fc tail and in vitro functionality tests were performed including viability, apoptosis and proliferation assays in human AML cell lines and primary patient samples. Using these in vitro models, its anti-tumor effect was evaluated as a single agent, and in combination with standard of care agents venetoclax or cytarabine. Results: Based on its cell binding potential, cross-reactivity, nanomolar affinity and GAS6/AXL blocking capacity, we selected sdAb20 for further evaluation. Using SPECT/CT imaging, we observed tumor uptake of 99mTc-sdAb20 in mice with AXL-positive THP-1 or C1498 tumors. In THP-1 xenografts, an optimized protocol using pre-injection of cold sdAb20-Fc was required to maximize the tumor-to-background signal. Besides its diagnostic value, we observed a significant reduction in tumor cell proliferation and viability using sdAb20-Fc in vitro. Moreover, combining sdAb20-Fc and cytarabine synergistically induced apoptosis in human AML cell lines, while these effects were less clear when combined with venetoclax. Conclusions: Because of their diagnostic potential, sdAbs could be used to screen patients eligible for AXL-targeted therapy and to follow-up AXL expression during treatment and disease progression. When fused to an Fc-domain, sdAbs acquire additional therapeutic properties that can lead to a multidrug approach for the treatment of AXL-positive cancer patients.
Keywords: AXL; acute myeloid leukemia; nuclear imaging; single domain antibodies; therapy.
© The author(s).
Conflict of interest statement
Competing Interests: N.D. is co-founder and scientific consultant at Precirix NV and ABSCINT NV. All other authors declare no conflict of interest.
Figures





References
-
- Cancer.Net. Leukemia - Acute Myeloid - AML: Statistics. American Society of Clinical Oncology (ASCO) 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

