CRISPR-Cas9 library screening combined with an exosome-targeted delivery system addresses tumorigenesis/TMZ resistance in the mesenchymal subtype of glioblastoma
- PMID: 38773970
- PMCID: PMC11103500
- DOI: 10.7150/thno.92703
CRISPR-Cas9 library screening combined with an exosome-targeted delivery system addresses tumorigenesis/TMZ resistance in the mesenchymal subtype of glioblastoma
Abstract
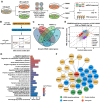
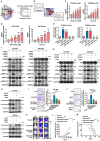
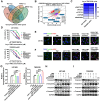
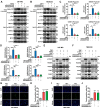
Rationale: The large-scale genomic analysis classifies glioblastoma (GBM) into three major subtypes, including classical (CL), proneural (PN), and mesenchymal (MES) subtypes. Each of these subtypes exhibits a varying degree of sensitivity to the temozolomide (TMZ) treatment, while the prognosis corresponds to the molecular and genetic characteristics of the tumor cell type. Tumors with MES features are predominantly characterized by the NF1 deletion/alteration, leading to sustained activation of the RAS and PI3K-AKT signaling pathways in GBM and tend to acquire drug resistance, resulting in the worst prognosis compared to other subtypes (PN and CL). Here, we used the CRISPR/Cas9 library screening technique to detect TMZ-related gene targets that might play roles in acquiring drug resistance, using overexpressed KRAS-G12C mutant GBM cell lines. The study identified a key therapeutic strategy to address the chemoresistance against the MES subtype of GBM. Methods: The CRISPR-Cas9 library screening was used to discover genes associated with TMZ resistance in the U87-KRAS (U87-MG which is overexpressed KRAS-G12C mutant) cells. The patient-derived GBM primary cell line TBD0220 was used for experimental validations in vivo and in vitro. Chromatin isolation by RNA purification (ChIRP) and chromatin immunoprecipitation (ChIP) assays were used to elucidate the silencing mechanism of tumor suppressor genes in the MES-GBM subtype. The small-molecule inhibitor EPIC-0412 was obtained through high-throughput screening. Transmission electron microscopy (TEM) was used to characterize the exosomes (Exos) secreted by GBM cells after TMZ treatment. Blood-derived Exos-based targeted delivery of siRNA, TMZ, and EPIC-0412 was optimized to tailor personalized therapy in vivo. Results: Using the genome-wide CRISPR-Cas9 library screening, we found that the ERBIN gene could be epigenetically regulated in the U87-KRAS cells. ERBIN overexpression inhibited the RAS signaling and downstream proliferation and invasion effects of GBM tumor cells. EPIC-0412 treatment inhibited tumor proliferation and EMT progression by upregulating the ERBIN expression both in vitro and in vivo. Genome-wide CRISPR-Cas9 screening also identified RASGRP1(Ras guanine nucleotide-releasing protein 1) and VPS28(Vacuolar protein sorting-associated protein 28) genes as synthetically lethal in response to TMZ treatment in the U87-KRAS cells. We found that RASGRP1 activated the RAS-mediated DDR pathway by promoting the RAS-GTP transformation. VPS28 promoted the Exos secretion and decreased intracellular TMZ concentration in GBM cells. The targeted Exos delivery system encapsulating drugs and siRNAs together showed a powerful therapeutic effect against GBM in vivo. Conclusions: We demonstrate a new mechanism by which ERBIN is epigenetically silenced by the RAS signaling in the MES subtype of GBM. Restoration of the ERBIN expression with EPIC-0412 significantly inhibits the RAS signaling downstream. RASGRP1 and VPS28 genes are associated with the promotion of TMZ resistance through RAS-GDP to RAS-GTP transformation and TMZ efflux, as well. A quadruple combination therapy based on a targeted Exos delivery system demonstrated significantly reduced tumor burden in vivo. Therefore, our study provides new insights and therapeutic approaches for regulating tumor progression and TMZ resistance in the MES-GBM subtype.
Keywords: CRISPR/Cas9; ERBIN; RASGRP1; VPS28; glioblastoma.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70:299–312. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

