The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review
- PMID: 38774006
- PMCID: PMC11103046
- DOI: 10.1016/j.jaccao.2024.01.007
The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review
Abstract
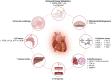
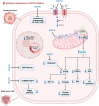
Sodium-glucose cotransporter-2 (SGLT2) inhibitors, originally approved for type 2 diabetes mellitus, have demonstrated efficacy in reducing cardiovascular events, particularly heart failure, in patients with and without diabetes. An intriguing research area involves exploring the potential application of SGLT2 inhibitors in cardio-oncology, aiming to mitigate the cardiovascular adverse events associated with anticancer treatments. These inhibitors present a unique dual nature, offering both cardioprotective effects and anticancer properties, conferring a double benefit for cardio-oncology patients. In this review, the authors first examine the established cardioprotective effects of SGLT2 inhibitors in heart failure and subsequently explore the existing body of evidence, including both preclinical and clinical studies, that supports the use of SGLT2 inhibitors in the context of cardio-oncology. The authors further discuss the mechanisms through which SGLT2 inhibitors protect against cardiovascular toxicity secondary to cancer treatment. Finally, they explore the potential anticancer effects of SGLT2 inhibitors along with their proposed mechanisms.
Keywords: SGLT2 inhibitors; anthracyclines; cancer; cardio-oncology; cardiotoxicity.
© 2024 The Authors.
Conflict of interest statement
Dr Zordoky is supported by the SURRGE Award from the University of Minnesota College of Pharmacy and by National Institutes of Health grant R01HL151740. Dr Dabour is supported by a scholarship from the Egyptian Ministry of Higher Education. Dr George is supported by the Fulbright Visiting Scholar Postdoctoral Program, U.S. Department of State, Bureau of Educational and Cultural Affairs. Dr Blaes is supported by the University of Minnesota Cancer Center grant (P30CA077598), and by National Institutes of Health grants R01CA267977, 1R01CA277714-01, R21AG080503, and R13CA278261. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. - PubMed
-
- Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. - PubMed
-
- Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. - PubMed
-
- McMurray J.J., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

