Anthracycline Cardiotoxicity Induces Progressive Changes in Myocardial Metabolism and Mitochondrial Quality Control: Novel Therapeutic Target
- PMID: 38774018
- PMCID: PMC11103041
- DOI: 10.1016/j.jaccao.2024.02.005
Anthracycline Cardiotoxicity Induces Progressive Changes in Myocardial Metabolism and Mitochondrial Quality Control: Novel Therapeutic Target
Abstract
Background: Anthracycline-induced cardiotoxicity (AIC) debilitates quality of life in cancer survivors. Serial characterizations are lacking of the molecular processes occurring with AIC.
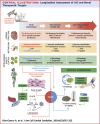
Objectives: The aim of this study was to characterize AIC progression in a mouse model from early (subclinical) to advanced heart failure stages, with an emphasis on cardiac metabolism and mitochondrial structure and function.
Methods: CD1 mice received 5 weekly intraperitoneal doxorubicin injections (5 mg/kg) and were followed by serial echocardiography for 15 weeks. At 1, 9, and 15 weeks after the doxorubicin injections, mice underwent fluorodeoxyglucose positron emission tomography, and hearts were extracted for microscopy and molecular analysis.
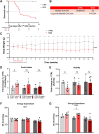
Results: Cardiac atrophy was evident at 1 week post-doxorubicin (left ventricular [LV] mass 117 ± 26 mg vs 97 ± 25 mg at baseline and 1 week, respectively; P < 0.001). Cardiac mass nadir was observed at week 3 post-doxorubicin (79 ± 16 mg; P = 0.002 vs baseline), remaining unchanged thereafter. Histology confirmed significantly reduced cardiomyocyte area (167 ± 19 μm2 in doxorubicin-treated mice vs 211 ± 26 μm2 in controls; P = 0.004). LV ejection fraction declined from week 6 post-doxorubicin (49% ± 9% vs 61% ± 9% at baseline; P < 0.001) until the end of follow-up at 15 weeks (43% ± 8%; P < 0.001 vs baseline). At 1 week post-doxorubicin, when LV ejection fraction remained normal, reduced cardiac metabolism was evident from down-regulated markers of fatty acid oxidation and glycolysis. Metabolic impairment continued to the end of follow-up in parallel with reduced mitochondrial adenosine triphosphate production. A transient early up-regulation of nutrient-sensing and mitophagy markers were observed, which was associated with mitochondrial enlargement. Later stages, when mitophagy was exhausted, were characterized by overt mitochondrial fragmentation.
Conclusions: Cardiac atrophy, global hypometabolism, early transient-enhanced mitophagy, biogenesis, and nutrient sensing constitute candidate targets for AIC prevention.
Keywords: anthracyclines; cancer; cardio-oncology; cardiotoxicity; doxorubicin; mitochondria.
© 2024 The Authors.
Conflict of interest statement
Dr Ibáñez is supported by the European Commission (grants ERC-CoG 819775 and H2020-HEALTH 945118), the Spanish Ministry of Science, Innovation and Universities (grant PID2022-140176OB-I00), and Comunidad de Madrid through the Red Madrileña de Nanomedicina en Imagen Molecular (grant P2022/BMD-7403 RENIM-CM). Dr Díaz-Guerra’s PhD fellowship is funded by the Spanish Association Against Cancer. Dr Oliver is a Ramón y Cajal fellow (grant RYC2020-028884-I) funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in Your Future.” Centro Nacional de Investigaciones Cardiovasculares is supported by Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures







References
-
- Lyon A.R., Lopez-Fernandez T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43:4229–4361. - PubMed
-
- Lopez-Sendon J., Alvarez-Ortega C., Zamora Aunon P., et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41:1720–1729. - PubMed
LinkOut - more resources
Full Text Sources

