Learning capabilities to resolve tilt-translation ambiguity in goldfish
- PMID: 38774058
- PMCID: PMC11106485
- DOI: 10.3389/fneur.2024.1304496
Learning capabilities to resolve tilt-translation ambiguity in goldfish
Abstract
Introduction: Spatial orientation refers to the perception of relative location and self-motion in space. The accurate formation of spatial orientation is essential for animals to survive and interact safely with their environment. The formation of spatial orientation involves the integration of sensory inputs from the vestibular, visual, and proprioceptive systems. Vestibular organs function as specialized head motion sensors, providing information regarding angular velocity and linear acceleration via the semicircular canals and otoliths, respectively. However, because forces arising from the linear acceleration (translation) and inclination relative to the gravitational axis (tilt) are equivalent, they are indistinguishable by accelerometers, including otoliths. This is commonly referred to as the tilt - translation ambiguity, which can occasionally lead to the misinterpretation of translation as a tilt. The major theoretical frameworks addressing this issue have proposed that the interpretation of tilt versus translation may be contingent on an animal's previous experiences of motion. However, empirical confirmation of this hypothesis is lacking.
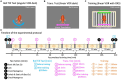
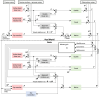
Methods: In this study, we conducted a behavioral experiment using goldfish to investigate how an animal's motion experience influences its interpretation of tilt vs. translation. We examined a reflexive eye movement called the vestibulo-ocular reflex (VOR), which compensatory-rotates the eyes in response to head motion and is known to reflect an animal's three-dimensional head motion estimate.
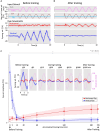
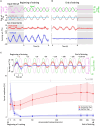
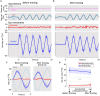
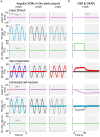
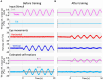
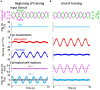
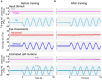
Results: We demonstrated that the VORs of naïve goldfish do not differentiate between translation and tilt at 0.5 Hz. However, following prolonged visual-translation training, which provided appropriate visual stimulation in conjunction with translational head motion, the VORs were capable of distinguishing between the two types of head motion within 3 h. These results were replicated using the Kalman filter model of spatial orientation, which incorporated the variable variance of process noise corresponding to the accumulated motion experience.
Discussion: Based on these experimental and computational findings, we discuss the neural mechanism underlying the resolution of tilt-translation ambiguity within a context analogous to, yet distinct from, previous cross-axis VOR adaptations.
Keywords: Kalman filter model; adaptive learning ability; gravito-inertial axis; somatogravic illusion; spatial orientation; state space model; translation ambiguity; vestibulo-ocular reflex.
Copyright © 2024 Tadokoro, Shinji, Yamanaka and Hirata.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Mehlman ML, Taube JS. In vivo electrophysiological approaches for studying head direction cells. Handb Behav Neurosci. (2018) 28:169–87. doi: 10.1016/B978-0-12-812028-6.00009-4 - DOI
-
- Gillingham KK, Wolfe JW. Spatial orientation in flight In: DeHart RL, editor. Fundamentals of aerospace medicine, vol. 5. Philadelphia: Lea & Febiger; (1985). 76–381.
LinkOut - more resources
Full Text Sources

