Increased Vasoactive Intestinal Peptide (VIP) in polycystic ovary syndrome patients undergoing IVF
- PMID: 38774232
- PMCID: PMC11106456
- DOI: 10.3389/fendo.2024.1331282
Increased Vasoactive Intestinal Peptide (VIP) in polycystic ovary syndrome patients undergoing IVF
Abstract
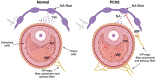
Introduction: Polycystic ovary syndrome (PCOS) is a common multifactorial and polygenic disorder of the endocrine system, affecting up to 20% of women in reproductive age with a still unknown etiology. Follicular fluid (FF) represents an environment for the normal development of follicles rich in metabolites, hormones and neurotransmitters, but in some instances of PCOS the composition can be different. Vasoactive intestinal peptide (VIP) is an endogenous autonomic neuropeptide involved in follicular atresia, granulosa cell physiology and steroidogenesis.
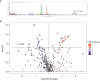
Methods: ELISA assays were performed to measure VIP and estradiol levels in human follicular fluids, while AMH, FSH, LH, estradiol and progesterone in the plasma were quantified by chemiluminescence. UHPLC/QTOF was used to perform the untargeted metabolomic analysis.
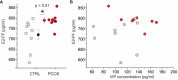
Results: Our ELISA and metabolomic results show: i) an increased concentration of VIP in follicular fluid of PCOS patients (n=9) of about 30% with respect to control group (n=10) (132 ± 28 pg/ml versus 103 ± 26 pg/ml, p=0,03) in women undergoing in vitro fertilization (IVF), ii) a linear positive correlation (p=0.05, r=0.45) between VIP concentration and serum Anti-Müllerian Hormone (AMH) concentration and iii) a linear negative correlation between VIP and noradrenaline metabolism. No correlation between VIP and estradiol (E2) concentration in follicular fluid was found. A negative correlation was found between VIP and noradrenaline metabolite 3,4-dihydroxyphenylglycolaldehyde (DOPGAL) in follicular fluids.
Conclusion: VIP concentration in follicular fluids was increased in PCOS patients and a correlation was found with noradrenaline metabolism indicating a possible dysregulation of the sympathetic reflex in the ovarian follicles. The functional role of VIP as noradrenergic modulator in ovarian physiology and PCOS pathophysiology was discussed.
Keywords: In Vitro Fertilization (IVF); PCOS (polycystic ovarian syndrome); VIP (vasoactive intestinal peptide); follicular fluid; metabolomic; noradrenalin (NA); ovarian innervation.
Copyright © 2024 Sallicandro, Gliozheni, Feudi, Sabbatini, Pellegrino, Alabed, Baldini, Gerli, Alviggi, Cascardi, Cicinelli, Malvasi and Fioretti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

