Quantitative systems pharmacology-based exploration of relevant anti-amyloid therapy challenges in clinical practice
- PMID: 38774587
- PMCID: PMC11106679
- DOI: 10.1002/trc2.12474
Quantitative systems pharmacology-based exploration of relevant anti-amyloid therapy challenges in clinical practice
Abstract
Introduction: Addressing practical challenges in clinical practice after the recent approvals of amyloid antibodies in Alzheimer's disease (AD) will benefit more patients. However, generating these answers using clinical trials or real-world evidence is not practical, nor feasible.
Methods: Here we use a Quantitative Systems Pharmacology (QSP) computational model of amyloid aggregation dynamics, well validated with clinical data on biomarkers and amyloid-related imaging abnormality-edema (ARIA-E) liability of six amyloid antibodies in clinical trials to explore various clinical practice challenges.
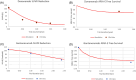
Results: Treatment duration to reach amyloid negativity ranges from 12 to 44, 16 to 40, and 6 to 20 months for lecanemab, aducanumab, and donanemab, respectively, for baseline central amyloid values between 50 and 200 Centiloids (CL). Changes in plasma cerebrospinal fluid Aβ42 and the plasma Aβ42/ Aβ40 ratio-fluid biomarkers to detect central amyloid negativity-is greater for lecanemab than for aducanumab and donanemab, indicating that these fluid amyloid biomarkers are only suitable for lecanemab. After reaching amyloid negativity an optimal maintenance schedule consists of a 24-month, 48-month and 64-month interval for 10 mg/kg (mpk) lecanemab, 10 mpk aducanumab, and 20 mpk donanemab, respectively, to keep central amyloid negative for 10 years. Cumulative ARIA-E liability could be reduced to almost half by introducing a drug holiday in the first months. For patients experiencing ARIA-E, restarting treatment with a conservative titration strategy resulted in an additional delay ranging between 3 and 4 months (donanemab), 5 months (lecanemab), and up to 7 months (aducanumab) for reaching amyloid negativity, depending upon the timing of the incident. Clinical trial designs for Down syndrome patients suggested the same rank order for central amyloid reduction, but higher ARIA-E liability especially for donanemab, which can be significantly mitigated by adopting a longer titration period.
Discussion: This QSP platform could support clinical practice challenges to optimize real-world treatment paradigms for new and existing amyloid drugs.
© 2024 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors do not have any conflicts of interest. Author disclosures are available in the Supporting Information.
Figures





References
-
- Angioni D, Hansson O, Bateman RJ, et al. Can we use blood biomarkers as entry criteria and for monitoring drug treatment effects in clinical trials? A report from the EU/US CTAD task force. J Prev Alzheimers Dis. 2023;10:418‐425. - PubMed
-
- Howe MD, Britton KJ, Joyce HE, et al. Initial experiences with amyloid‐related imaging abnormalities in patients receiving Aducanumab following accelerated approval. J Prev Alzheimers Dis. 2023;10:765‐770. - PubMed
LinkOut - more resources
Full Text Sources
