This is a preprint.
mRNA nuclear clustering leads to a difference in mutant huntingtin mRNA and protein silencing by siRNAs in vivo
- PMID: 38774633
- PMCID: PMC11106801
- DOI: 10.1101/2024.04.24.590997
mRNA nuclear clustering leads to a difference in mutant huntingtin mRNA and protein silencing by siRNAs in vivo
Update in
-
mRNA Nuclear Clustering Leads to a Difference in Mutant Huntingtin mRNA and Protein Silencing by siRNAs In Vivo.Nucleic Acid Ther. 2024 Aug;34(4):164-172. doi: 10.1089/nat.2024.0027. Epub 2024 Jul 18. Nucleic Acid Ther. 2024. PMID: 39023561 Free PMC article.
Abstract
Huntington's disease (HD) is an autosomal dominant neurodegenerative disease caused by CAG repeat expansion in the first exon of the huntingtin gene (HTT). Oligonucleotide therapeutics, such as short interfering RNA (siRNA), reduce levels of huntingtin mRNA and protein in vivo and are considered a viable therapeutic strategy. However, the extent to which they silence HTT mRNA in the nucleus is not established. We synthesized siRNA cross-reactive to mouse (wild-type) Htt and human (mutant) HTT in a di-valent scaffold and delivered to two mouse models of HD. In both models, di-valent siRNA sustained lowering of wild-type Htt, but not mutant HTT mRNA expression in striatum and cortex. Near-complete silencing of both mutant HTT protein and wild-type Htt protein was observed in both models. Subsequent fluorescent in situ hybridization (FISH) analysis shows that di-valent siRNA acts predominantly on cytoplasmic mutant HTT transcripts, leaving clustered mutant HTT transcripts in the nucleus largely intact in treated HD mouse brains. The observed differences between mRNA and protein levels, exaggerated in the case of extended repeats, might apply to other repeat-associated neurological disorders.
Keywords: Huntington’s Disease; mRNA aggregation; nuclear localization; siRNA.
Figures

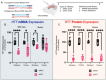

References
-
- McColgan P. and Tabrizi S.J. (2018) Huntington’s disease: a clinical review. Eur. J. Neurol., 25, 24–34. - PubMed
-
- MacDonald M.E., Ambrose C.M., Duyao M.P., Myers R.H., Lin C., Srinidhi L., Barnes G., Taylor S.A., James M., et al. (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell, 72, 971–983. - PubMed
-
- Ross C.A. and Tabrizi S.J. (2011) Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol., 10, 83–98. - PubMed
-
- Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium. Electronic address: gusella@helix.mgh.harvard.edu and Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium (2019) CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell, 178, 887–900.e14. - PMC - PubMed
-
- Slow E.J., van Raamsdonk J., Rogers D., Coleman S.H., Graham R.K., Deng Y., Oh R., Bissada N., Hossain S.M., et al. (2003) Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum. Mol. Genet., 12, 1555–1567. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
