Bacille Calmette-Guérin vaccination to prevent febrile and respiratory illness in adults (BRACE): secondary outcomes of a randomised controlled phase 3 trial
- PMID: 38774675
- PMCID: PMC11106519
- DOI: 10.1016/j.eclinm.2024.102616
Bacille Calmette-Guérin vaccination to prevent febrile and respiratory illness in adults (BRACE): secondary outcomes of a randomised controlled phase 3 trial
Abstract
Background: Bacille Calmette-Guérin (BCG) vaccination has off-target (non-specific) effects that are associated with protection against unrelated infections and decreased all-cause mortality in infants. We aimed to determine whether BCG vaccination prevents febrile and respiratory infections in adults.
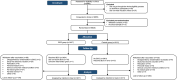
Methods: This randomised controlled phase 3 trial was done in 36 healthcare centres in Australia, Brazil, the Netherlands, Spain, and the United Kingdom. Healthcare workers were randomised to receive BCG-Denmark (single 0.1 ml intradermal injection) or no BCG in a 1:1 ratio using a web-based procedure, stratified by stage, site, age, and presence of co-morbidity. The difference in occurrence of febrile or respiratory illness were measured over 12 months (prespecified secondary outcome) using the intention-to-treat (ITT) population. This trial is registered with ClinicalTrials.gov, NCT04327206.
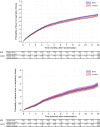
Findings: Between March 30, 2020, and April 1, 2021, 6828 healthcare workers were randomised to BCG-Denmark (n = 3417) or control (n = 3411; no intervention or placebo) groups. The 12-month adjusted estimated risk of ≥1 episode of febrile or respiratory illness was 66.8% in the BCG group (95% CI 65.3%-68.2%), compared with 63.4% in the control group (95% CI 61.8%-65.0%), a difference of +3.4 percentage points (95% CI +1.3% to +5.5%; p 0.002). The adjusted estimated risk of a severe episode (defined as being incapacitated for ≥3 consecutive days or hospitalised) was 19.4% in the BCG group (95% CI 18.0%-20.7%), compared with 18.8% in the control group (95% CI 17.4%-20.2%) a difference of +0.6 percentage points (95% CI -1.3% to +2.5%; p 0.6). Both groups had a similar number of episodes of illness, pneumonia, and hospitalisation. There were three deaths, all in the control group. There were no safety concerns following BCG vaccination.
Interpretation: In contrast to the beneficial off-target effects reported following neonatal BCG in infants, a small increased risk of symptomatic febrile or respiratory illness was observed in the 12 months following BCG vaccination in adults. There was no evidence of a difference in the risk of severe disease.
Funding: Bill & Melinda Gates Foundation, Minderoo Foundation, Sarah and Lachlan Murdoch, the Royal Children's Hospital Foundation, Health Services Union NSW, the Peter Sowerby Foundation, SA Health, the Insurance Advisernet Foundation, the NAB Foundation, the Calvert-Jones Foundation, the Modara Pines Charitable Foundation, the UHG Foundation Pty Ltd, Epworth Healthcare, the National Health and Medical Research Council, the Swiss National Science Foundation and individual donors.
Keywords: Bacille Calmette-Guérin (BCG) vaccine; Health personnel; Heterologous; Immunity; Placebo; Primary prevention; Randomised controlled trial.
© 2024 The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/and declare: the trial is financially supported by the Foundations listed in the Funding section. Authors disclose funding support over the past 36 months: National Health and Medical Research Council (NHMRC) Ideas Grant (NM), Investigator Grant (NC); Melbourne Children's Clinician-Scientist Fellowship Grant (KPP); Institutional support for research grants, presentations, meeting attendance and participation on Scientific Advisory Boards related to pertussis, RSV, pneumococcal, meningococcal and COVID-19 diseases from GlaxoSmithKline, Merck Sharpe & Dohme, Pfizer, Clover Biopharmaceuticals and Resvinet Foundation (PCR); grant support from Sanofi, MSD & CEPI for COVID-19 vaccine/drug research (JC). PCR has participated on Astra Zeneca COVID-19 Scientific Advisory Board and has a bacteriotherapy patent pending. JC participates on Latin American data safety monitoring/advisory boards for mRNA-1273 (Modern/Zodiac), RSV maternal vaccine (Pfizer), Qdenga vaccine (Takeda), Nirmatrelvir/Ritonavir-Paxlovid (Pfizer) and recently presented to Foro Latinoamericano para Asesores Médicos en Vacunas (Pfizer). NW has participated in the Covalia COVID-19 DNA vaccine trial in an advisory/data safety monitoring board capacity. KG is a member of the Royal Children's Hospital (RCH) Human Research Ethics Committee (the primary ethics committee providing approval for the BRACE trial) and Director of Research Operations at RCH; she abstained from all discussion, voting, approval and review related to the BRACE trial.
Figures
References
-
- Wardhana, Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43(3):185–190. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

