Effect of timed dosing of usual antihypertensives according to patient chronotype on cardiovascular outcomes: the Chronotype sub-study cohort of the Treatment in Morning versus Evening (TIME) study
- PMID: 38774676
- PMCID: PMC11106533
- DOI: 10.1016/j.eclinm.2024.102633
Effect of timed dosing of usual antihypertensives according to patient chronotype on cardiovascular outcomes: the Chronotype sub-study cohort of the Treatment in Morning versus Evening (TIME) study
Abstract
Background: Timing drug administration to endogenous circadian rhythms may enhance treatment efficacy. In the Chronotype sub-study of the Treatment in Morning versus Evening (TIME) clinical trial we examined whether timing of usual antihypertensive medications according to patient chronotype (a behavioural marker of personal circadian rhythm) may influence clinical cardiovascular outcomes.
Methods: This was a cohort sub-study of TIME, a prospective, randomised, open-label, blinded-endpoint, UK clinical trial of morning versus evening dosing of usual antihypertensive medications and cardiovascular outcomes. On August 3rd, 2020, all active TIME participants were invited to complete a validated chronotype questionnaire. Chronotype was quantitatively assessed as the mid sleep time on free days corrected for sleep debt on workdays (MSFsc). We analysed associations between chronotype and antihypertensive dosing time and explored their combined effect on cardiovascular outcomes (a composite endpoint of hospitalisation for non-fatal myocardial infarction (MI) or non-fatal stroke, and single components) using proportional hazard time-to-event models adjusted for baseline covariates. These were used to specifically test for interactions between dosing time and chronotype.
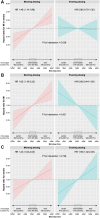
Findings: Between August 3, 2020, and March 31, 2021, 5358 TIME participants completed the online questionnaire. 2778 were previously randomised to morning dosing and 2580 to evening dosing of their usual antihypertensives. Chronotype was symmetrically distributed around a median MSFsc of 3:07 am. The composite endpoint increased for later MSFsc (later chronotype) dosed in the morning but not in those dosed in the evening (hazard ratios 1.46 [95% CI 1.14-1.86] and 0.96 [95% CI 0.70-1.30] per hour of MSFsc, respectively; interaction p = 0.036). Later chronotype was associated with increased risk of hospitalisation for non-fatal MI in the morning dosing group, and reduced risk in the evening dosing group (hazard ratios 1.62 [95% CI 1.18-2.22] and 0.66 [95% CI 0.44-1.00] per hour of MSFsc, respectively; interaction p < 0.001). No interaction between chronotype and antihypertensive dosing time was observed for stroke events.
Interpretation: Alignment of dosing time of usual antihypertensives with personal chronotype could lower the incidence of non-fatal MI compared to a 'misaligned' dosing time regimen. Future studies are warranted to establish whether synchronizing administration time of antihypertensive therapy with individual chronotype reduces risk of MI.
Funding: The TIME study was funded by the British Heart Foundation (CS/14/1/30659) with support from the British and Irish Hypertension Society.
Keywords: Antihypertensive; Cardiovascular outcomes; Chronotype; Dosing time; Hypertension; Personalised chronotherapy.
© 2024 The Author(s).
Conflict of interest statement
FP, KAD, SVM, RF, DR, and RM report no conflicts of interest. CV reports to be a paid scientific consultant of the National Institutes of Mental Health, USA. CV is now an employee of IQVIA GmbH, Frankfurt am Main, Germany. AR reports travel expenses from Informa, and unpaid membership of an NIHR Trial Steering Committee. IM reports research grants from British Heart Foundation for the submitted work, and research grants from Menarini, EMA, Sanofi, HDR UK, NIHR HTA and IMI, institutional consultancy income from AstraZeneca, and personal income from AstraZeneca, Amgen, Amarin, Novartis and NovoNordisk outside the submitted work. TM reports grants from Menarini/Ipsen/Teijin, NIHR HTA and MSD outside the submitted work and from British Heart Foundation for the submitted work and personal income for consultancy from Novartis and AstraZeneca outside the submitted work. TM and IM are trustees of the Scottish Heart and Arterial Risk Prevention (SHARP) Society. FPC reports a grant from NIHR i4i outside the submitted work, consultancies from Omron Healthcare, Menarini Int., Deloitte UK, and royalties from OUP for two books. He is also unpaid advisor to the World Health Organization for work unrelated to the subject of the present study.
Figures

Similar articles
-
Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial.Lancet. 2022 Oct 22;400(10361):1417-1425. doi: 10.1016/S0140-6736(22)01786-X. Epub 2022 Oct 11. Lancet. 2022. PMID: 36240838 Free PMC article. Clinical Trial.
-
Methods of a large prospective, randomised, open-label, blinded end-point study comparing morning versus evening dosing in hypertensive patients: the Treatment In Morning versus Evening (TIME) study.BMJ Open. 2016 Feb 9;6(2):e010313. doi: 10.1136/bmjopen-2015-010313. BMJ Open. 2016. PMID: 26861939 Free PMC article. Clinical Trial.
-
Circadian rhythms and decision-making: a review and new evidence from electroencephalography.Chronobiol Int. 2020 Apr;37(4):520-541. doi: 10.1080/07420528.2020.1715421. Epub 2020 Jan 31. Chronobiol Int. 2020. PMID: 32000532 Review.
-
Associations between chronotype, morbidity and mortality in the UK Biobank cohort.Chronobiol Int. 2018 Aug;35(8):1045-1053. doi: 10.1080/07420528.2018.1454458. Epub 2018 Apr 11. Chronobiol Int. 2018. PMID: 29642757 Free PMC article.
-
Timing of Antihypertensive Drug Therapy: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.Hypertension. 2023 Jul;80(7):1544-1554. doi: 10.1161/HYPERTENSIONAHA.122.20862. Epub 2023 May 22. Hypertension. 2023. PMID: 37212152
Cited by
-
Circadian attributes of neurological and psychiatric disorders as basis for their medication chronotherapy.Adv Drug Deliv Rev. 2025 Aug;223:115576. doi: 10.1016/j.addr.2025.115576. Epub 2025 Apr 3. Adv Drug Deliv Rev. 2025. PMID: 40187645 Review.
-
Circadian immune system in solid organ transplantation: a review article.Front Immunol. 2025 Mar 3;16:1556057. doi: 10.3389/fimmu.2025.1556057. eCollection 2025. Front Immunol. 2025. PMID: 40098968 Free PMC article. Review.
-
Nocturnal blood pressure: pathophysiology, measurement and clinical implications. Position paper of the European Society of Hypertension.J Hypertens. 2025 Aug 1;43(8):1296-1318. doi: 10.1097/HJH.0000000000004053. Epub 2025 Jun 12. J Hypertens. 2025. PMID: 40509714 Free PMC article. Review.
-
A Narrative Review on How Timing Matters: Circadian and Sleep Influences on Influenza Vaccine Induced Immunity.Vaccines (Basel). 2025 Aug 8;13(8):845. doi: 10.3390/vaccines13080845. Vaccines (Basel). 2025. PMID: 40872930 Free PMC article. Review.
-
Considering chronotype to improve hypertension management.EClinicalMedicine. 2024 Jul 26;75:102768. doi: 10.1016/j.eclinm.2024.102768. eCollection 2024 Sep. EClinicalMedicine. 2024. PMID: 39170940 Free PMC article. No abstract available.
References
-
- Millar-Craig M.W., Bishop C.N., Raftery E.B. Circadian variation of blood-pressure. Lancet. 1978;1(8068):795–797. - PubMed
-
- Manfredini R., Gallerani M., Portaluppi F., et al. Relationships of the circadian rhythms of thrombotic, ischemic, hemorrhagic, and arrhythmic events to blood pressure rhythms. Ann N Y Acad Sci. 1996;783:141–158. - PubMed
LinkOut - more resources
Full Text Sources

