METTL3 Deficiency Aggravates Hepatic Ischemia/Reperfusion Injury in Mice by Activating the MAPK Signaling Pathway
- PMID: 38774758
- PMCID: PMC11103385
- DOI: 10.7150/ijms.94177
METTL3 Deficiency Aggravates Hepatic Ischemia/Reperfusion Injury in Mice by Activating the MAPK Signaling Pathway
Abstract
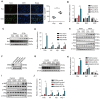
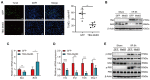
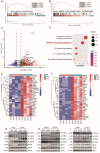
Background: Inflammatory responses, apoptosis, and oxidative stress, are key factors that contribute to hepatic ischemia/reperfusion (I/R) injury, which may lead to the failure of liver surgeries, such as hepatectomy and liver transplantation. The N6-methyladenosine (m6A) modification has been implicated in multiple biological processes, and its specific role and mechanism in hepatic I/R injury require further investigation. Methods: Dot blotting analysis was used to profile m6A levels in liver tissues at different reperfusion time points in hepatic I/R mouse models. Hepatocyte-specific METTL3 knockdown (HKD) mice were used to determine the function of METTL3 during hepatic I/R. RNA sequencing and western blotting were performed to assess the potential signaling pathways involved with the deficiency of METTL3. Finally, AAV8-TBG-METTL3 was injected through the tail vein to further elucidate the role of METTL3 in hepatic I/R injury. Results: The m6A modification levels and the expression of METTL3 were upregulated in mouse livers during hepatic I/R injury. METTL3 deficiency led to an exacerbated inflammatory response and increased cell death during hepatic I/R, whereas overexpression of METTL3 reduced the extent of liver injury. Bioinformatic analysis revealed that the MAPK pathway was significantly enriched in the livers of METTL3-deficient mice. METTL3 protected the liver from I/R injury, possibly by inhibiting the phosphorylation of JNK and ERK, but not P38. Conclusions: METTL3 deficiency aggravates hepatic I/R injury in mice by activating the MAPK signaling pathway. METTL3 may be a potential therapeutic target in hepatic I/R injury.
Keywords: Hepatic ischemia/reperfusion injury; MAPK.; METTL3; apoptosis; inflammatory response; m6A.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Wisse E, Braet F. et al. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol. 1996;24:100–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

