Differential regulation of lung homeostasis and silicosis by the TAM receptors MerTk and Axl
- PMID: 38774866
- PMCID: PMC11106457
- DOI: 10.3389/fimmu.2024.1380628
Differential regulation of lung homeostasis and silicosis by the TAM receptors MerTk and Axl
Abstract
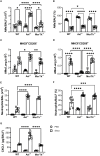
Introduction: TAM receptor-mediated efferocytosis plays an important function in immune regulation and may contribute to antigen tolerance in the lungs, a site with continuous cellular turnover and generation of apoptotic cells. Some studies have identified failures in efferocytosis as a common driver of inflammation and tissue destruction in lung diseases. Our study is the first to characterize the in vivo function of the TAM receptors, Axl and MerTk, in the innate immune cell compartment, cytokine and chemokine production, as well as the alveolar macrophage (AM) phenotype in different settings in the airways and lung parenchyma.
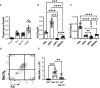
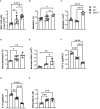
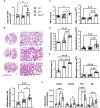
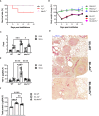
Methods: We employed MerTk and Axl defective mice to induce acute silicosis by a single exposure to crystalline silica particles (20 mg/50 μL). Although both mRNA levels of Axl and MerTk receptors were constitutively expressed by lung cells and isolated AMs, we found that MerTk was critical for maintaining lung homeostasis, whereas Axl played a role in the regulation of silica-induced inflammation. Our findings imply that MerTk and Axl differently modulated inflammatory tone via AM and neutrophil recruitment, phenotype and function by flow cytometry, and TGF-β and CXCL1 protein levels, respectively. Finally, Axl expression was upregulated in both MerTk-/- and WT AMs, confirming its importance during inflammation.
Conclusion: This study provides strong evidence that MerTk and Axl are specialized to orchestrate apoptotic cell clearance across different circumstances and may have important implications for the understanding of pulmonary inflammatory disorders as well as for the development of new approaches to therapy.
Keywords: airways homeostasis; alveolar macrophage; efferocytosis; immunoregulation; silicosis.
Copyright © 2024 Guimarães-Pinto, Leandro, Corrêa, Maia, Rodrigues, da Costa, Rafael Machado Ferreira, Claudio-Etienne, Siebenlist, He, Rigoni, Ferreira, Jannini-Sa, Matos-Guedes, Costa-da-Silva, Lopes, Silva, Kelsall and Filardy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

