OTULIN deficiency: focus on innate immune system impairment
- PMID: 38774872
- PMCID: PMC11106414
- DOI: 10.3389/fimmu.2024.1371564
OTULIN deficiency: focus on innate immune system impairment
Abstract
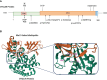
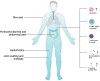
OTULIN deficiency is a complex disease characterized by a wide range of clinical manifestations, including skin rash, joint welling, lipodystrophy to pulmonary abscess, and sepsis shock. This disease is mechanistically linked to mutations in the OTULIN gene, resulting in an immune disorder that compromises the body's ability to effectively combat pathogens and foreign stimuli. The OTULIN gene is responsible for encoding a deubiquitinating enzyme crucial for hydrolyzing Met1-poly Ub chains, and its dysfunction leads to dysregulated immune responses. Patients with OTULIN deficiency often exhibit an increase in monocytes, including neutrophils and macrophages, along with inflammatory clinical features. The onset of symptoms typically occurs at an early age. However, individuals with OTULIN haploinsufficiency are particularly susceptible to life-threatening staphylococcal infections. Currently, the most effective treatment for patients with OTULIN biallelic mutations involves the use of TNF-blocking agents, which target the dysregulated immune response. In conclusion, OTULIN deficiency presents a complex clinical picture with diverse manifestations, attributed to mutations in the OTULIN gene. Understanding the underlying mechanisms is crucial for developing targeted therapeutic interventions to address this challenging condition. Further research into the pathophysiology of OTULIN deficiency is essential for improving clinical management and outcomes for affected individuals.
Keywords: OTULIN; autoinflammatory disorder; deubiquitinase; genetic deficiency; immunodeficient disorder.
Copyright © 2024 Dou, Jiang, Peng and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

