Puerarin mitigated LPS-ATP or HG-primed endothelial cells damage and diabetes-associated cardiovascular disease via ROS-NLRP3 signalling
- PMID: 38774996
- PMCID: PMC11109626
- DOI: 10.1111/jcmm.18239
Puerarin mitigated LPS-ATP or HG-primed endothelial cells damage and diabetes-associated cardiovascular disease via ROS-NLRP3 signalling
Abstract
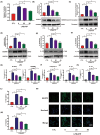
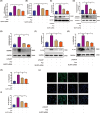
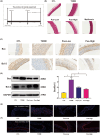
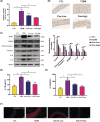
The occurrence and development of diabetic vascular diseases are closely linked to inflammation-induced endothelial dysfunction. Puerarin (Pue), the primary component of Pueraria lobata, possesses potent anti-inflammatory properties. However, its vasoprotective role remains elusive. Therefore, we investigated whether Pue can effectively protect against vascular damage induced by diabetes. In the study, Pue ameliorated lipopolysaccharide-adenosine triphosphate (LPS-ATP) or HG-primed cytotoxicity and apoptosis, while inhibited reactive oxygen species (ROS)-mediated NLR family pyrin domain containing 3 (NLRP3) inflammasome in HUVECs, as evidenced by significantly decreased ROS level, NOX4, Caspase-1 activity and expression of NLRP3, GSDMD, cleaved caspase-1, IL-1β and IL-18. Meanwhile, ROS inducer CoCI2 efficiently weakened the effects of Pue against LPS-ATP-primed pyroptosis. In addition, NLRP3 knockdown notably enhanced Pue's ability to suppress pyroptosis in LPS-ATP-primed HUVECs, whereas overexpression of NLRP3 reversed the inhibitory effects of Pue. Furthermore, Pue inhibited the expression of ROS and NLRP3 inflammasome-associated proteins on the aorta in type 2 diabetes mellitus rats. Our findings indicated that Pue might ameliorate LPS-ATP or HG-primed damage in HUVECs by inactivating the ROS-NLRP3 signalling pathway.
Keywords: Puerarin; ROS; diabetic vasculopathy; pyroptosis.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures


Similar articles
-
Neferine inhibits LPS-ATP-induced endothelial cell pyroptosis via regulation of ROS/NLRP3/Caspase-1 signaling pathway.Inflamm Res. 2019 Sep;68(9):727-738. doi: 10.1007/s00011-019-01256-6. Epub 2019 Jun 6. Inflamm Res. 2019. PMID: 31172209
-
Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes.J Diabetes Res. 2019 Feb 17;2019:8151836. doi: 10.1155/2019/8151836. eCollection 2019. J Diabetes Res. 2019. PMID: 30911553 Free PMC article.
-
Puerarin inhibits hyperglycemia-induced inter-endothelial junction through suppressing endothelial Nlrp3 inflammasome activation via ROS-dependent oxidative pathway.Phytomedicine. 2019 Mar 1;55:310-319. doi: 10.1016/j.phymed.2018.10.013. Epub 2018 Oct 11. Phytomedicine. 2019. PMID: 30385134
-
Puerarin reduces diabetic nephropathy-induced podocyte pyroptosis by modulating the SIRT1/NLRP3/caspase-1 pathway.Mol Cell Endocrinol. 2025 Jan 1;595:112409. doi: 10.1016/j.mce.2024.112409. Epub 2024 Nov 6. Mol Cell Endocrinol. 2025. PMID: 39515602
-
Resveratrol improves gasdermin D-mediated pyroptosis of vascular endothelial cells induced by a high-fat diet and palmitic acid possibly via the SIRT1-p66Shc-NLRP3 pathway.J Nutr Biochem. 2025 Jun;140:109890. doi: 10.1016/j.jnutbio.2025.109890. Epub 2025 Mar 5. J Nutr Biochem. 2025. PMID: 40054674
Cited by
-
Comprehensive treatment of diabetic endothelial dysfunction based on pathophysiological mechanism.Front Med (Lausanne). 2025 Feb 28;12:1509884. doi: 10.3389/fmed.2025.1509884. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40093018 Free PMC article. Review.
-
Puerarin as a Phytochemical Modulator of Gastrointestinal Homeostasis in Livestock: Molecular Mechanisms and Translational Applications.Antioxidants (Basel). 2025 Jun 19;14(6):756. doi: 10.3390/antiox14060756. Antioxidants (Basel). 2025. PMID: 40563388 Free PMC article. Review.
-
Traditional Chinese medicine in the prevention of diabetes mellitus and cardiovascular complications: mechanisms and therapeutic approaches.Front Pharmacol. 2025 Apr 11;16:1511701. doi: 10.3389/fphar.2025.1511701. eCollection 2025. Front Pharmacol. 2025. PMID: 40290429 Free PMC article. Review.
-
Therapeutic potential of omentin-1 in preeclampsia: enhancing fetal outcomes, vascular function, and reducing inflammation.Exp Anim. 2025 Apr 20;74(2):216-228. doi: 10.1538/expanim.24-0092. Epub 2024 Dec 7. Exp Anim. 2025. PMID: 39647913 Free PMC article.
-
Qideng Mingmu Capsules Ameliorates Retinal Neovascularization by Regulating Ang/Tie2 Signaling Pathway.Chin J Integr Med. 2025 Jun 10. doi: 10.1007/s11655-025-4131-3. Online ahead of print. Chin J Integr Med. 2025. PMID: 40490600
References
-
- Rigalleau V, Larroumet A, Mohammedi K, et al. Comment on Gange et al. incidence of proliferative diabetic retinopathy and other neovascular sequelae at 5 years following diagnosis of type 2 diabetes. Diabetes care 2021;44:2518–2526. Diabetes Care. 2022;45:e60. - PubMed
-
- Mahdi A, Kövamees O, Checa A, et al. Arginase inhibition improves endothelial function in patients with type 2 diabetes mellitus despite intensive glucose‐lowering therapy. J Intern Med. 2018;284:388‐398. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

