The Spermine Oxidase/Spermine Axis Coordinates ATG5-Mediated Autophagy to Orchestrate Renal Senescence and Fibrosis
- PMID: 38775007
- PMCID: PMC11304251
- DOI: 10.1002/advs.202306912
The Spermine Oxidase/Spermine Axis Coordinates ATG5-Mediated Autophagy to Orchestrate Renal Senescence and Fibrosis
Abstract
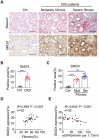
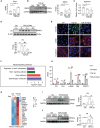
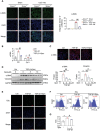
Decreased plasma spermine levels are associated with kidney dysfunction. However, the role of spermine in kidney disease remains largely unknown. Herein, it is demonstrated that spermine oxidase (SMOX), a key enzyme governing polyamine metabolism, is predominantly induced in tubular epithelium of human and mouse fibrotic kidneys, alongside a reduction in renal spermine content in mice. Moreover, renal SMOX expression is positively correlated with kidney fibrosis and function decline in patients with chronic kidney disease. Importantly, supplementation with exogenous spermine or genetically deficient SMOX markedly improves autophagy, reduces senescence, and attenuates fibrosis in mouse kidneys. Further, downregulation of ATG5, a critical component of autophagy, in tubular epithelial cells enhances SMOX expression and reduces spermine in TGF-β1-induced fibrogenesis in vitro and kidney fibrosis in vivo. Mechanically, ATG5 readily interacts with SMOX under physiological conditions and in TGF-β1-induced fibrogenic responses to preserve cellular spermine levels. Collectively, the findings suggest SMOX/spermine axis is a potential novel therapy to antagonize renal fibrosis, possibly by coordinating autophagy and suppressing senescence.
Keywords: SMOX; autophagy; cell senescence; renal fibrosis; spermine.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Djudjaj S., Boor P., Mol. Aspects Med. 2019, 65, 16. - PubMed
-
- Zhao X., Kwan J. Y. Y., Yip K., Liu P. P., Liu F. F., Nat. Rev. Drug Discovery. 2020, 19, 57. - PubMed
-
- Verissimo T., Faivre A., Rinaldi A., Lindenmeyer M., Delitsikou V., Veyrat‐Durebex C., Heckenmeyer C., Fernandez M., Berchtold L., Dalga D., Cohen C., Naesens M., Ricksten S. E., Martin P. Y., Pugin J., Merlier F., Haupt K., Rutkowski J. M., Moll S., Cippa P. E., Legouis D., De Seigneux S., J. Am. Soc. Nephrol. 2022, 33, 810. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
