Aging impairs cold-induced beige adipogenesis and adipocyte metabolic reprogramming
- PMID: 38775132
- PMCID: PMC11111218
- DOI: 10.7554/eLife.87756
Aging impairs cold-induced beige adipogenesis and adipocyte metabolic reprogramming
Abstract
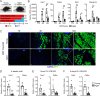
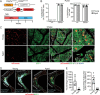
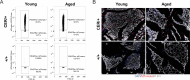
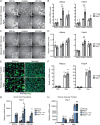
The energy-burning capability of beige adipose tissue is a potential therapeutic tool for reducing obesity and metabolic disease, but this capacity is decreased by aging. Here, we evaluate the impact of aging on the profile and activity of adipocyte stem and progenitor cells (ASPCs) and adipocytes during the beiging process in mice. We found that aging increases the expression of Cd9 and other fibro-inflammatory genes in fibroblastic ASPCs and blocks their differentiation into beige adipocytes. Fibroblastic ASPC populations from young and aged mice were equally competent for beige differentiation in vitro, suggesting that environmental factors suppress adipogenesis in vivo. Examination of adipocytes by single nucleus RNA-sequencing identified compositional and transcriptional differences in adipocyte populations with aging and cold exposure. Notably, cold exposure induced an adipocyte population expressing high levels of de novo lipogenesis (DNL) genes, and this response was severely blunted in aged animals. We further identified Npr3, which encodes the natriuretic peptide clearance receptor, as a marker gene for a subset of white adipocytes and an aging-upregulated gene in adipocytes. In summary, this study indicates that aging blocks beige adipogenesis and dysregulates adipocyte responses to cold exposure and provides a resource for identifying cold and aging-regulated pathways in adipose tissue.
Keywords: UCP1; aging; beige adipocyte; beige adipogenesis; cell biology; cold exposure; developmental biology; mouse.
© 2023, Holman et al.
Conflict of interest statement
CH, AS, RC, LC, EF, CJ, LT, ER, PS No competing interests declared
Figures







Update of
-
Aging impairs cold-induced beige adipogenesis and adipocyte metabolic reprogramming.bioRxiv [Preprint]. 2024 Feb 1:2023.03.20.533514. doi: 10.1101/2023.03.20.533514. bioRxiv. 2024. Update in: Elife. 2024 May 22;12:RP87756. doi: 10.7554/eLife.87756. PMID: 36993336 Free PMC article. Updated. Preprint.
Comment in
-
Becoming less beige with age.Elife. 2023 Jul 7;12:e89700. doi: 10.7554/eLife.89700. Elife. 2023. PMID: 37417552 Free PMC article.
References
-
- Barreau C, Labit E, Guissard C, Rouquette J, Boizeau ML, Gani Koumassi S, Carrière A, Jeanson Y, Berger-Müller S, Dromard C, Plouraboué F, Casteilla L, Lorsignol A. Regionalization of browning revealed by whole subcutaneous adipose tissue imaging. Obesity. 2016;24:1081–1089. doi: 10.1002/oby.21455. - DOI - PubMed
MeSH terms
Associated data
- Actions
Grants and funding
- T32 DK007314/NH/NIH HHS/United States
- P30-DK19525/NH/NIH HHS/United States
- R01 DK120982/DK/NIDDK NIH HHS/United States
- R01 DK121801/DK/NIDDK NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- DK120982/NH/NIH HHS/United States
- RC2 DK116691/DK/NIDDK NIH HHS/United States
- T32 HD083185/HD/NICHD NIH HHS/United States
- DK116691/NH/NIH HHS/United States
- T32 HD083185/NH/NIH HHS/United States
- P30 DK135043/DK/NIDDK NIH HHS/United States
- DK121801/NH/NIH HHS/United States
- T32 DK007314/DK/NIDDK NIH HHS/United States
- P30 DK019525/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

