Lentivirus-mediated gene therapy corrects ribosomal biogenesis and shows promise for Diamond Blackfan anemia
- PMID: 38775150
- PMCID: PMC11141922
- DOI: 10.1172/jci.insight.171650
Lentivirus-mediated gene therapy corrects ribosomal biogenesis and shows promise for Diamond Blackfan anemia
Abstract
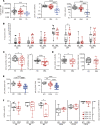
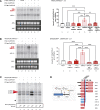
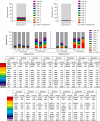
This study lays the groundwork for future lentivirus-mediated gene therapy in patients with Diamond Blackfan anemia (DBA) caused by mutations in ribosomal protein S19 (RPS19), showing evidence of a new safe and effective therapy. The data show that, unlike patients with Fanconi anemia (FA), the hematopoietic stem cell (HSC) reservoir of patients with DBA was not significantly reduced, suggesting that collection of these cells should not constitute a remarkable restriction for DBA gene therapy. Subsequently, 2 clinically applicable lentiviral vectors were developed. In the former lentiviral vector, PGK.CoRPS19 LV, a codon-optimized version of RPS19 was driven by the phosphoglycerate kinase promoter (PGK) already used in different gene therapy trials, including FA gene therapy. In the latter one, EF1α.CoRPS19 LV, RPS19 expression was driven by the elongation factor alpha short promoter, EF1α(s). Preclinical experiments showed that transduction of DBA patient CD34+ cells with the PGK.CoRPS19 LV restored erythroid differentiation, and demonstrated the long-term repopulating properties of corrected DBA CD34+ cells, providing evidence of improved erythroid maturation. Concomitantly, long-term restoration of ribosomal biogenesis was verified using a potentially novel method applicable to patients' blood cells, based on ribosomal RNA methylation analyses. Finally, in vivo safety studies and proviral insertion site analyses showed that lentivirus-mediated gene therapy was nontoxic.
Keywords: Gene therapy; Genetic diseases; Hematology; Hematopoietic stem cells; Therapeutics.
Conflict of interest statement
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

