Human Epidemiology and Response to SARS-CoV-2 (HEROS): objectives, design, and enrollment results of a 12-city remote observational surveillance study of households with children, using direct-to-participant methods
- PMID: 38775275
- PMCID: PMC12096296
- DOI: 10.1093/aje/kwae077
Human Epidemiology and Response to SARS-CoV-2 (HEROS): objectives, design, and enrollment results of a 12-city remote observational surveillance study of households with children, using direct-to-participant methods
Abstract
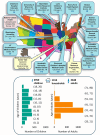
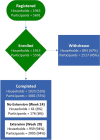
The Human Epidemiology and Response to SARS-CoV-2 (HEROS) Study is a prospective, multicity, 6-month incidence study conducted from May 2020 to February 2021. The objectives were to identify risk factors for SARS-CoV-2 infection and household transmission among children and people with asthma and allergic diseases, and to use the host nasal transcriptome sampled longitudinally to understand infection risk and sequelae at the molecular level. To overcome challenges of clinical study implementation due to the coronavirus pandemic, this surveillance study used direct-to-participant methods to remotely enroll and prospectively follow eligible children who are participants in other National Institutes of Health-funded pediatric research studies and their household members. Households participated in weekly surveys and biweekly nasal sampling regardless of symptoms. The aim of this report is to widely share the methods and study instruments and to describe the rationale, design, execution, logistics, and characteristics of a large, observational, household-based, remote cohort study of SARS-CoV-2 infection and transmission in households with children. The study enrolled a total of 5598 individuals, including 1913 principal participants (children), 1913 primary caregivers, 729 secondary caregivers, and 1043 other household children. This study was successfully implemented without necessitating any in-person research visits and provides an approach for rapid execution of clinical research. Trial registration: ClinicalTrials.gov. Identifier: NCT04375761.
Keywords: COVID-19; public health; remote methods; surveillance.
Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health 2024.
Conflict of interest statement
The authors declare no relevant conflicts of interest.
Figures

Update of
-
Human Epidemiology and RespOnse to SARS-CoV-2 (HEROS): Objectives, Design and Enrollment Results of a 12-City Remote Observational Surveillance Study of Households with Children using Direct-to-Participant Methods.medRxiv [Preprint]. 2022 Jul 10:2022.07.09.22277457. doi: 10.1101/2022.07.09.22277457. medRxiv. 2022. Update in: Am J Epidemiol. 2024 Oct 7;193(10):1329-1338. doi: 10.1093/aje/kwae077. PMID: 35860216 Free PMC article. Updated. Preprint.
References
-
- US Census Bureau, US Department of Commerce . 2020 Census operational plan. A new design for the 21st century. US Census Bureau website. 2015. Accessed November 21, 2021. https://www2.census.gov/programs-surveys/decennial/2020/program-manageme...
Publication types
MeSH terms
Associated data
Grants and funding
- U01 AI110397/AI/NIAID NIH HHS/United States
- R01 AI127507/AI/NIAID NIH HHS/United States
- R01 HL137192/HL/NHLBI NIH HHS/United States
- UH3 OD023282/OD/NIH HHS/United States
- UM1 AI114271/AI/NIAID NIH HHS/United States
- AI024156/Henry Ford Health System
- UG3 OD023282/OD/NIH HHS/United States
- U19 AI 095227-S2/Vanderbilt University
- U10 HL109172/HL/NHLBI NIH HHS/United States
- 3UM1AI114271-06S1/Columbia University Medical Center
- R56 AI050681/AI/NIAID NIH HHS/United States
- 1UM2AI117870/Rho
- P30 ES036084/ES/NIEHS NIH HHS/United States
- 3UM1AI14271-07S1/St. Louis Children's Hospital
- 3R01AI130348-04S1/Lurie Children's Hospital
- UM2 AI117870/AI/NIAID NIH HHS/United States
- U54 AI117804/AI/NIAID NIH HHS/United States
- 1UL1TR001430/Boston Children's Hospital
- R01 AI130348/AI/NIAID NIH HHS/United States
- P01 AI089473/AI/NIAID NIH HHS/United States
- R01 AI050681/AI/NIAID NIH HHS/United States
- 5UM1AI114271/Children's National Health System
- 3UM1AI151958-02S1/National Jewish Health
- 3U54AI117804-06S1/Children's Hospital Colorado
- U19 AI070235/AI/NIAID NIH HHS/United States
- U19 AI104317/AI/NIAID NIH HHS/United States
- 3UM1AI114271-06S1/Cincinnati Children's Hospital Medical Center
- UM1 AI151958/AI/NIAID NIH HHS/United States
- R01AI127507/Mass General Hospital
- UL1 TR002243/TR/NCATS NIH HHS/United States
- U19 AI095227/AI/NIAID NIH HHS/United States
- R01 AI051598/AI/NIAID NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- K24 AI106822/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

