Long-term effects of ipragliflozin and pioglitazone on metabolic dysfunction-associated steatotic liver disease in patients with type 2 diabetes: 5 year observational follow-up of a randomized, 24 week, active-controlled trial: Effect of ipragliflozin in MASLD
- PMID: 38775319
- PMCID: PMC11363141
- DOI: 10.1111/jdi.14246
Long-term effects of ipragliflozin and pioglitazone on metabolic dysfunction-associated steatotic liver disease in patients with type 2 diabetes: 5 year observational follow-up of a randomized, 24 week, active-controlled trial: Effect of ipragliflozin in MASLD
Abstract
Aims/introduction: We conducted a 5 year post-trial monitoring study of our previous randomized 24 week, open-label, active-controlled trial that showed beneficial effects of ipragliflozin on metabolic dysfunction-associated steatotic liver disease (MASLD), identical to those of pioglitazone.
Materials and methods: In our previous trial, 66 patients with MASLD and type 2 diabetes were randomly assigned to receive either ipragliflozin (n = 32) or pioglitazone (n = 34). Upon its conclusion, 61 patients were monitored for 5 years for outcome measures of MASLD, glycemic, and metabolic parameters. Differences between the two groups were analyzed at baseline, 24 weeks, and 5 years; changes in outcome measures from baseline were also evaluated.
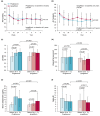
Results: At 5 years, the mean liver-to-spleen attenuation ratio increased by 0.20 (from 0.78 ± 0.24 to 0.98 ± 0.20) in the ipragliflozin group and by 0.26 (from 0.76 ± 0.26 to 1.02 ± 0.20) in the pioglitazone group (P = 0.363). Similarly, ipragliflozin and pioglitazone significantly improved serum aminotransferase, HbA1c, and fasting plasma glucose levels over 5 years. In the ipragliflozin group, significant reductions in body weight and visceral fat area observed at 24 weeks were sustained throughout the 5 years (-4.0%, P = 0.0075 and -7.6%, P = 0.045, respectively). Moreover, ipragliflozin significantly reduced the values of fibrosis markers (serum ferritin and FIB-4 index), was well tolerated, and had a higher continuation rate for 5 years compared with pioglitazone.
Conclusions: Ipragliflozin and pioglitazone improved MASLD and glycemic parameters over 5 years. In the ipragliflozin group, significant reductions in body weight and visceral fat mass persisted for 5 years.
Keywords: Metabolic dysfunction‐associated steatotic liver disease; Non‐alcoholic fatty liver disease; Sodium‐glucose cotransporter 2 inhibitors.
© 2024 The Author(s). Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
D.I. has received lecture fees from Astellas. No other potential conflicts of interest relevant to this article were reported.
Approval of the research protocol: This research protocol received ethical approval from the institutional review boards of all of the participating facilities in Japan (no. OGAWARIN047 [including affiliated clinics], no. 2021–04).
Informed consent: All participants provided written informed consent before enrollment in the intervention trial. In addition, informed consent was obtained in the form of opt‐out on the websites in the post‐interventional observational study.
Registry and the registration no. of the study/trial: This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000040611), 16 June 2020.
Animal studies: N/A.
Figures
References
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. - PubMed
-
- Lomonaco R, Ortiz‐Lopez C, Orsak B, et al. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology 2011; 54: 837–845. - PubMed
-
- Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta‐analysis. J Hepatol 2019; 71: 793–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

