Azithromycin resistance in Escherichia coli and Salmonella from food-producing animals and meat in Europe
- PMID: 38775752
- PMCID: PMC11215539
- DOI: 10.1093/jac/dkae161
Azithromycin resistance in Escherichia coli and Salmonella from food-producing animals and meat in Europe
Abstract
Objectives: To characterize the genetic basis of azithromycin resistance in Escherichia coli and Salmonella collected within the EU harmonized antimicrobial resistance (AMR) surveillance programme in 2014-18 and the Danish AMR surveillance programme in 2016-19.
Methods: WGS data of 1007 E. coli [165 azithromycin resistant (MIC > 16 mg/L)] and 269 Salmonella [29 azithromycin resistant (MIC > 16 mg/L)] were screened for acquired macrolide resistance genes and mutations in rplDV, 23S rRNA and acrB genes using ResFinder v4.0, AMRFinder Plus and custom scripts. Genotype-phenotype concordance was determined for all isolates. Transferability of mef(C)-mph(G)-carrying plasmids was assessed by conjugation experiments.
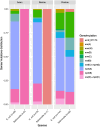
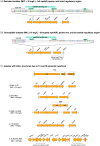
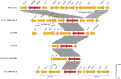
Results: mph(A), mph(B), mef(B), erm(B) and mef(C)-mph(G) were detected in E. coli and Salmonella, whereas erm(C), erm(42), ere(A) and mph(E)-msr(E) were detected in E. coli only. The presence of macrolide resistance genes, alone or in combination, was concordant with the azithromycin-resistant phenotype in 69% of isolates. Distinct mph(A) operon structures were observed in azithromycin-susceptible (n = 50) and -resistant (n = 136) isolates. mef(C)-mph(G) were detected in porcine and bovine E. coli and in porcine Salmonella enterica serovar Derby and Salmonella enterica 1,4, [5],12:i:-, flanked downstream by ISCR2 or TnAs1 and associated with IncIγ and IncFII plasmids.
Conclusions: Diverse azithromycin resistance genes were detected in E. coli and Salmonella from food-producing animals and meat in Europe. Azithromycin resistance genes mef(C)-mph(G) and erm(42) appear to be emerging primarily in porcine E. coli isolates. The identification of distinct mph(A) operon structures in susceptible and resistant isolates increases the predictive power of WGS-based methods for in silico detection of azithromycin resistance in Enterobacterales.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
-
- WHO . The WHO list of Critically Important Antimicrobials. 2019. https://www.who.int/groups/advisory-group-on-the-who-list-of-critically-....

