AMPK regulates phagophore-to-autophagosome maturation
- PMID: 38775785
- PMCID: PMC11110907
- DOI: 10.1083/jcb.202309145
AMPK regulates phagophore-to-autophagosome maturation
Abstract
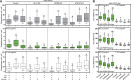
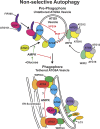
Autophagy is an important metabolic pathway that can non-selectively recycle cellular material or lead to targeted degradation of protein aggregates or damaged organelles. Autophagosome formation starts with autophagy factors accumulating on lipid vesicles containing ATG9. These phagophores attach to donor membranes, expand via ATG2-mediated lipid transfer, capture cargo, and mature into autophagosomes, ultimately fusing with lysosomes for their degradation. Autophagy can be activated by nutrient stress, for example, by a reduction in the cellular levels of amino acids. In contrast, how autophagy is regulated by low cellular ATP levels via the AMP-activated protein kinase (AMPK), an important therapeutic target, is less clear. Using live-cell imaging and an automated image analysis pipeline, we systematically dissect how nutrient starvation regulates autophagosome biogenesis. We demonstrate that glucose starvation downregulates autophagosome maturation by AMPK-mediated inhibition of phagophore tethering to donor membrane. Our results clarify AMPKs regulatory role in autophagy and highlight its potential as a therapeutic target to reduce autophagy.
© 2024 Barnaba et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
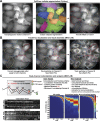

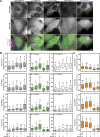


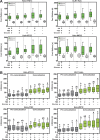
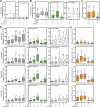





Update of
-
AMPK Regulates Phagophore-to-Autophagosome Maturation.bioRxiv [Preprint]. 2023 Sep 30:2023.09.28.559981. doi: 10.1101/2023.09.28.559981. bioRxiv. 2023. Update in: J Cell Biol. 2024 Aug 5;223(8):e202309145. doi: 10.1083/jcb.202309145. PMID: 37808644 Free PMC article. Updated. Preprint.
References
-
- Amaravadi, R.K., Kimmelman A.C., and Debnath J.. 2019. Targeting autophagy in cancer: Recent advances and future directions. Cancer Discov. 9:1167–1181. 10.1158/2159-8290.CD-19-0292 - DOI - PMC - PubMed
-
- Bakula, D., Müller A.J., Zuleger T., Takacs Z., Franz-Wachtel M., Thost A.-K., Brigger D., Tschan M.P., Frickey T., Robenek H., et al. . 2017. WIPI3 and WIPI4 β-propellers are scaffolds for LKB1-AMPK-TSC signalling circuits in the control of autophagy. Nat. Commun. 8:15637. 10.1038/ncomms15637 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

