Genetic Complexities of Cerebral Small Vessel Disease, Blood Pressure, and Dementia
- PMID: 38776079
- PMCID: PMC11112447
- DOI: 10.1001/jamanetworkopen.2024.12824
Genetic Complexities of Cerebral Small Vessel Disease, Blood Pressure, and Dementia
Abstract
Importance: Vascular disease is a treatable contributor to dementia risk, but the role of specific markers remains unclear, making prevention strategies uncertain.
Objective: To investigate the causal association between white matter hyperintensity (WMH) burden, clinical stroke, blood pressure (BP), and dementia risk, while accounting for potential epidemiologic biases.
Design, setting, and participants: This study first examined the association of genetically determined WMH burden, stroke, and BP levels with Alzheimer disease (AD) in a 2-sample mendelian randomization (2SMR) framework. Second, using population-based studies (1979-2018) with prospective dementia surveillance, the genetic association of WMH, stroke, and BP with incident all-cause dementia was examined. Data analysis was performed from July 26, 2020, through July 24, 2022.
Exposures: Genetically determined WMH burden and BP levels, as well as genetic liability to stroke derived from genome-wide association studies (GWASs) in European ancestry populations.
Main outcomes and measures: The association of genetic instruments for WMH, stroke, and BP with dementia was studied using GWASs of AD (defined clinically and additionally meta-analyzed including both clinically diagnosed AD and AD defined based on parental history [AD-meta]) for 2SMR and incident all-cause dementia for longitudinal analyses.
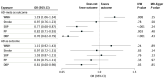
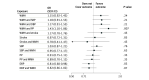
Results: In 2SMR (summary statistics-based) analyses using AD GWASs with up to 75 024 AD cases (mean [SD] age at AD onset, 75.5 [4.4] years; 56.9% women), larger WMH burden showed evidence for a causal association with increased risk of AD (odds ratio [OR], 1.43; 95% CI, 1.10-1.86; P = .007, per unit increase in WMH risk alleles) and AD-meta (OR, 1.19; 95% CI, 1.06-1.34; P = .008), after accounting for pulse pressure for the former. Blood pressure traits showed evidence for a protective association with AD, with evidence for confounding by shared genetic instruments. In the longitudinal (individual-level data) analyses involving 10 699 incident all-cause dementia cases (mean [SD] age at dementia diagnosis, 74.4 [9.1] years; 55.4% women), no significant association was observed between larger WMH burden and incident all-cause dementia (hazard ratio [HR], 1.02; 95% CI, 1.00-1.04; P = .07). Although all exposures were associated with mortality, with the strongest association observed for systolic BP (HR, 1.04; 95% CI, 1.03-1.06; P = 1.9 × 10-14), there was no evidence for selective survival bias during follow-up using illness-death models. In secondary analyses using polygenic scores, the association of genetic liability to stroke, but not genetically determined WMH, with dementia outcomes was attenuated after adjusting for interim stroke.
Conclusions: These findings suggest that WMH is a primary vascular factor associated with dementia risk, emphasizing its significance in preventive strategies for dementia. Future studies are warranted to examine whether this finding can be generalized to non-European populations.
Conflict of interest statement
Figures


Update of
-
Complexities of cerebral small vessel disease, blood pressure, and dementia relationship: new insights from genetics.medRxiv [Preprint]. 2023 Aug 13:2023.08.08.23293761. doi: 10.1101/2023.08.08.23293761. medRxiv. 2023. Update in: JAMA Netw Open. 2024 May 1;7(5):e2412824. doi: 10.1001/jamanetworkopen.2024.12824. PMID: 37790435 Free PMC article. Updated. Preprint.
References
-
- Wang H, Naghavi M, Allen C, et al. ; GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459-1544. doi: 10.1016/S0140-6736(16)31012-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- N01HC85080/HL/NHLBI NIH HHS/United States
- R01 AG023629/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- N01HC85082/HL/NHLBI NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- N01HC85086/HL/NHLBI NIH HHS/United States
- R01 AG031287/AG/NIA NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- N01HC85081/HL/NHLBI NIH HHS/United States
- U01 HL080295/HL/NHLBI NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- R01 HL120393/HL/NHLBI NIH HHS/United States
- R01 AG020098/AG/NIA NIH HHS/United States
- R01 HL103612/HL/NHLBI NIH HHS/United States
- R01 AG049607/AG/NIA NIH HHS/United States
- K23 NS110927/NS/NINDS NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- N01HC55222/HL/NHLBI NIH HHS/United States
- R01 AG054076/AG/NIA NIH HHS/United States
- N01HC85079/HL/NHLBI NIH HHS/United States
- N01HC85083/HL/NHLBI NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- R01 AG033193/AG/NIA NIH HHS/United States
- R01 AG015928/AG/NIA NIH HHS/United States
- U01 HL130114/HL/NHLBI NIH HHS/United States
- R01 HL087652/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

