Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation Across Mental Disorders: A Systematic Review and Dose-Response Meta-Analysis
- PMID: 38776083
- PMCID: PMC11112448
- DOI: 10.1001/jamanetworkopen.2024.12616
Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation Across Mental Disorders: A Systematic Review and Dose-Response Meta-Analysis
Erratum in
-
Errors in Byline and Author Affiliations.JAMA Netw Open. 2024 Jul 1;7(7):e2424292. doi: 10.1001/jamanetworkopen.2024.24292. JAMA Netw Open. 2024. PMID: 38958984 Free PMC article. No abstract available.
Abstract
Importance: Noninvasive brain stimulation (NIBS) interventions have been shown to be efficacious in several mental disorders, but the optimal dose stimulation parameters for each disorder are unknown.
Objective: To define NIBS dose stimulation parameters associated with the greatest efficacy in symptom improvement across mental disorders.
Data sources: Studies were drawn from an updated (to April 30, 2023) previous systematic review based on a search of PubMed, OVID, and Web of Knowledge.
Study selection: Randomized clinical trials were selected that tested transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) for any mental disorder in adults aged 18 years or older.
Data extraction and synthesis: Two authors independently extracted the data. A 1-stage dose-response meta-analysis using a random-effects model was performed. Sensitivity analyses were conducted to test robustness of the findings. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Main outcomes and measures: The main outcome was the near-maximal effective doses of total pulses received for TMS and total current dose in coulombs for tDCS.
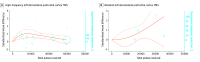
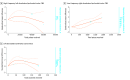
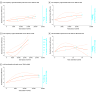
Results: A total of 110 studies with 4820 participants (2659 men [61.4%]; mean [SD] age, 42.3 [8.8] years) were included. The following significant dose-response associations emerged with bell-shaped curves: (1) in schizophrenia, high-frequency (HF) TMS on the left dorsolateral prefrontal cortex (LDLPFC) for negative symptoms (χ2 = 9.35; df = 2; P = .009) and TMS on the left temporoparietal junction for resistant hallucinations (χ2 = 36.52; df = 2; P < .001); (2) in depression, HF-DLPFC TMS (χ2 = 14.49; df = 2; P < .001); (3) in treatment-resistant depression, LDLPFC tDCS (χ2 = 14.56; df = 2; P < .001); and (4) in substance use disorder, LDLPFC tDCS (χ2 = 33.63; df = 2; P < .001). The following significant dose-response associations emerged with plateaued or ascending curves: (1) in depression, low-frequency (LF) TMS on the right DLPFC (RDLPFC) with ascending curve (χ2 = 25.67; df = 2; P = .001); (2) for treatment-resistant depression, LF TMS on the bilateral DLPFC with ascending curve (χ2 = 5.86; df = 2; P = .004); (3) in obsessive-compulsive disorder, LF-RDLPFC TMS with ascending curve (χ2 = 20.65; df = 2; P < .001) and LF TMS on the orbitofrontal cortex with a plateaued curve (χ2 = 15.19; df = 2; P < .001); and (4) in posttraumatic stress disorder, LF-RDLPFC TMS with ascending curve (χ2 = 54.15; df = 2; P < .001). Sensitivity analyses confirmed the main findings.
Conclusions and relevance: The study findings suggest that NIBS yields specific outcomes based on dose parameters across various mental disorders and brain regions. Clinicians should consider these dose parameters when prescribing NIBS. Additional research is needed to prospectively validate the findings in randomized, sham-controlled trials and explore how other parameters contribute to the observed dose-response association.
Conflict of interest statement
Figures

References
-
- Lõokene M, Markov N, Nikander M, Neuvonen T, Dilkov D. Reduction of symptoms in patients with major depressive disorder after transcranial direct current stimulation treatment: a real-world study. J Affect Disord Rep. 2022;8:100347. doi: 10.1016/j.jadr.2022.100347 - DOI
-
- Razza LB, Afonso Dos Santos L, Borrione L, et al. Appraising the effectiveness of electrical and magnetic brain stimulation techniques in acute major depressive episodes: an umbrella review of meta-analyses of randomized controlled trials. Braz J Psychiatry. 2021;43(5):514-524. doi: 10.1590/1516-4446-2020-1169 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

