Transcatheter or Surgical Replacement for Failed Bioprosthetic Aortic Valves
- PMID: 38776106
- PMCID: PMC11112500
- DOI: 10.1001/jamacardio.2024.1049
Transcatheter or Surgical Replacement for Failed Bioprosthetic Aortic Valves
Abstract
Importance: The use of valve-in-valve (ViV) transcatheter aortic valve replacement (TAVR) has been rapidly expanding as an alternative treatment to redo surgical aortic valve replacement (SAVR) for failed bioprosthetic valves despite limited long-term data.
Objective: To assess mortality and morbidity in patients undergoing intervention for failed bioprosthetic SAVR.
Design, setting, and participants: This was a retrospective population-based cohort analysis conducted between January 1, 2015, and December 31, 2020, with a median (IQR) follow-up time of 2.3 (1.1-4.0) years. A total of 1771 patients with a history of bioprosthetic SAVR who underwent ViV-TAVR or redo SAVR in California, New York, and New Jersey were included. Data were obtained from the California Department of Health Care Access and Information, the New York Statewide Planning and Research Cooperative System, and the New Jersey Discharge Data Collection System. Exclusion criteria included undergoing TAVR or redo SAVR within 5 years from initial SAVR, as well as infective endocarditis, concomitant surgical procedures, and out-of-state residency. Propensity matching yielded 375 patient pairs. Data were analyzed from January to December 2023.
Interventions: ViV-TAVR vs redo SAVR.
Main outcomes and measurements: The primary outcome was all-cause mortality. Secondary outcomes were stroke, heart failure hospitalization, reoperation, major bleeding, acute kidney failure, new pacemaker insertion, and infective endocarditis.
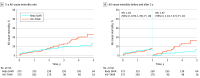
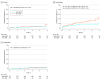
Results: From 2015 through 2020, the proportion of patients undergoing ViV-TAVR vs redo SAVR increased from 159 of 451 (35.3%) to 498 or 797 (62.5%). Of 1771 participants, 653 (36.9%) were female, and the mean (SD) age was 74.4 (11.3) years. Periprocedural mortality and stroke rates were similar between propensity-matched groups. The ViV-TAVR group had lower periprocedural rates of major bleeding (2.4% vs 5.1%; P = .05), acute kidney failure (1.3% vs 7.2%; P < .001), and new pacemaker implantations (3.5% vs 10.9%; P < .001). The 5-year all-cause mortality rate was 23.4% (95% CI, 15.7-34.1) in the ViV-TAVR group and 13.3% (95% CI, 9.2-18.9) in the redo SAVR group. In a landmark analysis, no difference in mortality was observed up to 2 years (hazard ratio, 1.03; 95% CI, 0.59-1.78), but after 2 years, ViV-TAVR was associated with higher mortality (hazard ratio, 2.97; 95% CI, 1.18-7.47) as well as with a higher incidence of heart failure hospitalization (hazard ratio, 3.81; 95% CI, 1.57-9.22). There were no differences in 5-year incidence of stroke, reoperation, major bleeding, or infective endocarditis.
Conclusions and relevance: Compared with redo SAVR, ViV-TAVR was associated with a lower incidence of periprocedural complications and a similar incidence of all-cause mortality through 2 years' follow-up. However, ViV-TAVR was associated with higher rates of late mortality and heart failure hospitalization. These findings may be influenced by residual confounding and require adjudication in a randomized clinical trial.
Conflict of interest statement
Figures
Comment on
-
Management Challenges for Bioprosthetic Aortic Valve Failure.JAMA Cardiol. 2024 Jul 1;9(7):639-640. doi: 10.1001/jamacardio.2024.1041. JAMA Cardiol. 2024. PMID: 38776100 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

