The oral nucleoside prodrug GS-5245 is efficacious against SARS-CoV-2 and other endemic, epidemic, and enzootic coronaviruses
- PMID: 38776389
- PMCID: PMC11333937
- DOI: 10.1126/scitranslmed.adj4504
The oral nucleoside prodrug GS-5245 is efficacious against SARS-CoV-2 and other endemic, epidemic, and enzootic coronaviruses
Abstract
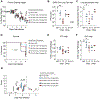
Despite the wide availability of several safe and effective vaccines that prevent severe COVID-19, the persistent emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) that can evade vaccine-elicited immunity remains a global health concern. In addition, the emergence of SARS-CoV-2 VOCs that can evade therapeutic monoclonal antibodies underscores the need for additional, variant-resistant treatment strategies. Here, we characterize the antiviral activity of GS-5245, obeldesivir (ODV), an oral prodrug of the parent nucleoside GS-441524, which targets the highly conserved viral RNA-dependent RNA polymerase (RdRp). We show that GS-5245 is broadly potent in vitro against alphacoronavirus HCoV-NL63, SARS-CoV, SARS-CoV-related bat-CoV RsSHC014, Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-2 WA/1, and the highly transmissible SARS-CoV-2 BA.1 Omicron variant. Moreover, in mouse models of SARS-CoV, SARS-CoV-2 (WA/1 and Omicron B1.1.529), MERS-CoV, and bat-CoV RsSHC014 pathogenesis, we observed a dose-dependent reduction in viral replication, body weight loss, acute lung injury, and pulmonary function with GS-5245 therapy. Last, we demonstrate that a combination of GS-5245 and main protease (Mpro) inhibitor nirmatrelvir improved outcomes in vivo against SARS-CoV-2 compared with the single agents. Together, our data support the clinical evaluation of GS-5245 against coronaviruses that cause or have the potential to cause human disease.
Figures

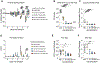




Update of
-
Efficacy of the oral nucleoside prodrug GS-5245 (Obeldesivir) against SARS-CoV-2 and coronaviruses with pandemic potential.bioRxiv [Preprint]. 2023 Jun 28:2023.06.27.546784. doi: 10.1101/2023.06.27.546784. bioRxiv. 2023. Update in: Sci Transl Med. 2024 May 22;16(748):eadj4504. doi: 10.1126/scitranslmed.adj4504. PMID: 37425890 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

