Gain-of-function mutations of TRPV4 acting in endothelial cells drive blood-CNS barrier breakdown and motor neuron degeneration in mice
- PMID: 38776392
- PMCID: PMC11316273
- DOI: 10.1126/scitranslmed.adk1358
Gain-of-function mutations of TRPV4 acting in endothelial cells drive blood-CNS barrier breakdown and motor neuron degeneration in mice
Abstract
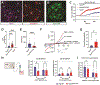
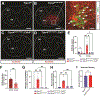
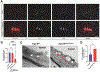
Blood-CNS barrier disruption is a hallmark of numerous neurological disorders, yet whether barrier breakdown is sufficient to trigger neurodegenerative disease remains unresolved. Therapeutic strategies to mitigate barrier hyperpermeability are also limited. Dominant missense mutations of the cation channel transient receptor potential vanilloid 4 (TRPV4) cause forms of hereditary motor neuron disease. To gain insights into the cellular basis of these disorders, we generated knock-in mouse models of TRPV4 channelopathy by introducing two disease-causing mutations (R269C and R232C) into the endogenous mouse Trpv4 gene. TRPV4 mutant mice exhibited weakness, early lethality, and regional motor neuron loss. Genetic deletion of the mutant Trpv4 allele from endothelial cells (but not neurons, glia, or muscle) rescued these phenotypes. Symptomatic mutant mice exhibited focal disruptions of blood-spinal cord barrier (BSCB) integrity, associated with a gain of function of mutant TRPV4 channel activity in neural vascular endothelial cells (NVECs) and alterations of NVEC tight junction structure. Systemic administration of a TRPV4-specific antagonist abrogated channel-mediated BSCB impairments and provided a marked phenotypic rescue of symptomatic mutant mice. Together, our findings show that mutant TRPV4 channels can drive motor neuron degeneration in a non-cell autonomous manner by precipitating focal breakdown of the BSCB. Further, these data highlight the reversibility of TRPV4-mediated BSCB impairments and identify a potential therapeutic strategy for patients with TRPV4 mutations.
Figures

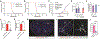
References
Publication types
MeSH terms
Substances
Grants and funding
- R35 NS122306/NS/NINDS NIH HHS/United States
- R61 HL154252/HL/NHLBI NIH HHS/United States
- R01 NS106008/NS/NINDS NIH HHS/United States
- R35 GM124824/GM/NIGMS NIH HHS/United States
- R01 NS132728/NS/NINDS NIH HHS/United States
- R33 HL154252/HL/NHLBI NIH HHS/United States
- T32 GM008752/GM/NIGMS NIH HHS/United States
- F31 NS105404/NS/NINDS NIH HHS/United States
- R01 NS104436/NS/NINDS NIH HHS/United States
- RF1 NS134549/NS/NINDS NIH HHS/United States
- R21 NS087579/NS/NINDS NIH HHS/United States
- R01 NS118014/NS/NINDS NIH HHS/United States
- U54 GM115677/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

