Targeted Ferroptosis-Immunotherapy Synergy: Enhanced Antiglioma Efficacy with Hybrid Nanovesicles Comprising NK Cell-Derived Exosomes and RSL3-Loaded Liposomes
- PMID: 38776411
- PMCID: PMC11164066
- DOI: 10.1021/acsami.4c04604
Targeted Ferroptosis-Immunotherapy Synergy: Enhanced Antiglioma Efficacy with Hybrid Nanovesicles Comprising NK Cell-Derived Exosomes and RSL3-Loaded Liposomes
Abstract
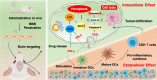
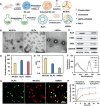
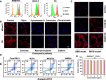
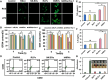
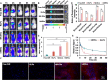
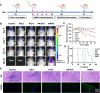
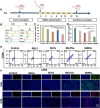
Ferroptosis therapy and immunotherapy have been widely used in cancer treatment. However, nonselective induction of ferroptosis in tumors is prone to immunosuppression, limiting the therapeutic effect of ferroptosis cancer treatment. To address this issue, this study reports a customized hybrid nanovesicle composed of NK cell-derived extracellular versicles and RSL3-loaded liposomes (hNRVs), aiming to establish a positive cycle between ferroptosis therapy and immunotherapy. Thanks to the enhanced permeability and retention effect and the tumor homing characteristics of NK exosomes, our data indicate that hNRVs can actively accumulate in tumors and enhance cellular uptake. FASL, IFN-γ, and RSL3 are released into the tumor microenvironment, where FASL derived from NK cells effectively lyses tumor cells. RSL3 downregulates the expression of GPX4 in the tumor, leading to the accumulation of LPO and ROS, and promotes ferroptosis in tumor cells. The accumulation of IFN-γ and TNF-α stimulates the maturation of dendritic cells and effectively induces the inactivation of GPX4, promoting lipid peroxidation, making them sensitive to ferroptosis and indirectly promoting the occurrence of ferroptosis. This study highlights the role of the customized hNRV platform in enhancing the effectiveness of synergistic treatment with selective delivery of ferroptosis inducers and immune activation against glioma without causing additional side effects on healthy organs.
Keywords: biomimetic hybrid nanovesicles; enhanced ferroptosis therapy; immunotherapy; natural killer cell; targeted delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Mitochondria-specific GPX4 inhibition enhances ferroptosis and antitumor immunity.J Control Release. 2025 Jul 10;383:113841. doi: 10.1016/j.jconrel.2025.113841. Epub 2025 May 13. J Control Release. 2025. PMID: 40373937
-
Induced Ferroptosis Pathway by Regulating Cellular Lipid Peroxidation With Peroxynitrite Generator for Reversing "Cold" Tumors.Small. 2024 Dec;20(49):e2404807. doi: 10.1002/smll.202404807. Epub 2024 Sep 16. Small. 2024. PMID: 39279600
-
Icariin promoted ferroptosis by activating mitochondrial dysfunction to inhibit colorectal cancer and synergistically enhanced the efficacy of PD-1 inhibitors.Phytomedicine. 2025 Jan;136:156224. doi: 10.1016/j.phymed.2024.156224. Epub 2024 Nov 23. Phytomedicine. 2025. PMID: 39642461
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
-
[Research progress on ferroptosis regulation in tumor immunity of hepatocellular carcinoma].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Dec 25;53(6):715-725. doi: 10.3724/zdxbyxb-2024-0117. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39694527 Free PMC article. Review. Chinese.
Cited by
-
Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders.Int J Mol Sci. 2025 Mar 11;26(6):2491. doi: 10.3390/ijms26062491. Int J Mol Sci. 2025. PMID: 40141135 Free PMC article. Review.
-
NK-derived exosomes in anti-tumor strategies.Med Oncol. 2025 Aug 9;42(9):418. doi: 10.1007/s12032-025-02965-1. Med Oncol. 2025. PMID: 40782186 Free PMC article. Review.
-
A Systematic Review of Nanoparticle-Mediated Ferroptosis in Glioma Therapy.Int J Nanomedicine. 2025 May 6;20:5779-5797. doi: 10.2147/IJN.S523008. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40351706 Free PMC article.
-
Therapeutic application of extracellular vesicles in human diseases.Mol Ther. 2025 May 7;33(5):2243-2251. doi: 10.1016/j.ymthe.2025.04.002. Epub 2025 Apr 3. Mol Ther. 2025. PMID: 40186351 Review.
-
Targeting Ferroptosis in Tumors: Novel Marine-Derived Compounds as Regulators of Lipid Peroxidation and GPX4 Signaling.Mar Drugs. 2025 Jun 19;23(6):258. doi: 10.3390/md23060258. Mar Drugs. 2025. PMID: 40559667 Free PMC article. Review.
References
-
- Fan Y.; Cui Y.; Hao W.; Chen M.; Liu Q.; Wang Y.; Yang M.; Li Z.; Gong W.; Song S.; Yang Y.; Gao C. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact Mater. 2021, 6, 4402–4414. 10.1016/j.bioactmat.2021.04.027. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

